Structure of Atom Class 9 Notes | Science | Term 2 |
Table of Contents
Structure of Atom Class 9 Notes | Science | Term 2 |
Structure of Atom Class 9 Science: The smallest particle of an element that can exist chemically is an atom. Dalton assumed that atom is indivisible. But experiments in late 1800s and early 1900s revealed that the atom is made up of three sub atomic particles- electrons, protons and neutrons. The atom is neutral in nature since it has equal number of negatively and positively charged particles.
Thomson’s Model of Atom
According to Thomson ‘s model of the atom:
An atom is basically a sphere of positive charge wherein negatively charged electrons are embedded.
The positive and negative charge in an atom are equal in magnitude that results in electrical neutral nature of an atom. Thus it has no overall positive or negative charge.
Thomson’s model of atom is quite similar to that of Christmas pudding. The electrons embedded in a sphere of positive charge are correlated with the dry fruits in a spherical Christmas pudding.
Rutherford’s Model of the Atom
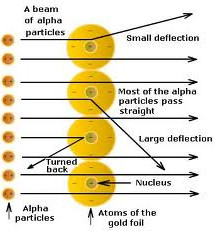
When fast moving Alpha particles are allowed to strike a very thin gold foil in vacuum than following observations were made:
- Most of the alpha particle pass straight through the gold foil without getting deflected from their original path.
- very few alpha particles are deflected through small angles.
- A very few Alpha particle completely rebound on hitting the gold foil and retraces their path.
Rutherford gave a nuclear model of atom that can be described as follows:
- An atom consists of A positively charged dense and very small nucleus containing all the protons and neutrons.
- It is considered that almost all the mass of atom is concentrated in the nucleus.
- Most of the atom is empty space.
- Nucleus is surrounded by a negatively charged electrons. These electrons revolve around the nucleus in circular path at a very high speed.
Drawback of Rutherford’s Model of the Atom
A major drawback of Rutherford’s model of atom is that it does not explain the stability of the atom. As Rutherford said that the negatively charged electrons revolve around the positively charged nucleus in circular path. Now we know that if an object moves in circular path then its motion is said to be accelerated. As per that electron radiate energy and its radius of path will become smaller. Does electron should fall into the nucleus but this does not happen.
Bohr’s Model of the Atom
This model was proposed by Neils Bohr in 1913.
The basic postulates of Bohr’s model of the atom are as follows:
- An atom consists of small heavy positively charged nucleus in the centre and the electrons revolve around it in a circular path called orbits.
- In a particular atom, the orbits in which the electron revolve are the discrete orbits having fixed radii and energy. These discrete orbits are called energy level or shells. Since the energy of the orbits is fixed these are also called stationary states.
- An electron can either lose energy or gain energy while revolving in any particular orbit. Thus atom is stable and does not collapse.
- Energy is lost or gained by an electron only when it jumps from one orbit to the another.
Atomic Number
The atomic parameter called atomic number was introduced by Moseley in 1913.
Atomic number of an element is equal to number of protons present in the nucleus of an atom of that element.
For example
Hydrogen atom and carbon atom have 1 and 6 protons respectively. Hence their atomic number will also be 1 and 6 respectively.
The number of protons in an atom is equal to the number of electrons since hole atom is electrically neutral.
The symbol ‘Z’ is generally used to represent atomic number of an element.
Atomic number of an element = number of protons present in the nucleus = number of electrons present in the extra nuclear part.
Representation of Atomic Number and Mass Number with the Symbol of Element
Atomic number and mass number are represented along with the symbol of the element. Atomic number is written on the lower side whereas mass number is written on the upper side of the symbol.
Electronic Configuration
Distribution of electron in shells (Orbits)
To write the electronic configuration we must know the following:
- Total number of electrons which is present in the atom
- Maximum number of electrons that can be present in each shell of the atom. This can be determined the formula 2n2 where n is the number of shell is counted from the nucleus.
- An atom become stable when its outermost shell has it electron or it has only one shell containing two electrons.
- Electrons do not go into a new shell until the inner shell is completely filled.
Valence Electrons
The electrons that are present in the outermost shell of an element are called as valence electrons in the outermost shell is commonly known as valence shell.
Significance of Valence Electron
- The valence electron of an atom are responsible for taking part in chemical changes.
- The valency of the atom can effectively be determined by the help of valence electrons.
- Similar chemical properties are possessed by the early as having the same number of valence electrons in their atoms.
- Different chemical properties are shown by elements having different number of valence electrons in their atoms.
Combining Capacity or Valency of Elements
Elements other than noble gases elements contain less than 8 electrons in their valence shells. These elements are generally chemically reactive and unstable. The tend to attain the stability by completing the outermost shell of the atom by losing, gaining or sharing electrons.
The number of electrons gained, lost or shared by the atom of the element in order to complete its octet or duplet is called the valency of the element.
| Name of Element | Symbol | Atomic Number | Number of Electrons | Number of Protons | Number of neutrons | Electronic Configuration
|
Valency | ||||
| Hydrogen | H | 1 | 1 | 1 | 0 |
|
1 | ||||
| Helium | He | 2 | 2 | 2 | 2 |
|
0 | ||||
| Lithium | Li | 3 | 3 | 3 | 4 |
|
1 | ||||
| Beryllium | Be | 4 | 4 | 4 | 5 |
|
2 | ||||
| Boron | B | 5 | 5 | 5 | 6 |
|
3 | ||||
| Carbon | C | 6 | 6 | 6 | 6 |
|
4 | ||||
| Nitrogen | N | 7 | 7 | 7 | 7 |
|
3 | ||||
| Oxygen | O | 8 | 8 | 8 | 8 |
|
2 | ||||
| Fluorine | F | 9 | 9 | 9 | 10 |
|
1 |
Isotopes
Atoms of the same element that possesses the same atomic number but have variable mass number are cold isotopes of that element.
Since all isotopes of an element have the same atomic number old isotope must have the same number of protons inside the nuclei. Also since different isotopes of an element have different mass number the number of neutrons present in the nuclei of isotopes of an element should be variable.
Isotopes of hydrogen- Hydrogen has three isotopes having mass number 1, 2 and 3 respectively. Atomic number of all the three isotopes is equal to 1. Protium, deuterium and tritium are the three isotopes of hydrogen.
Applications of Isotopes are as follows:
- Cracks in the metal castings can be located by isotopes.
- The treatment of thyroid disorder can effectively be done by isotope of iodine.
- Nuclear reactors in order to produce nuclear energy uses uranium isotope.
- Brain tumor can be removed by Cobalt isotope.
Isobars
These are simply the atoms of different elements with different atomic number that have the same atomic masses. These different elements are called as isobars. Example: Potassium and Calcium.
Questions / Answers of Structure of Atom
Question: Name a nucleus that does not contain neutron.
Answer: No neutrons are present in the nucleus of the hydrogen atom.
Question: What are some of the limitations of JJ thomson’s model of atom.
Answer: Thomson’s model explained that atoms are electrically neutral but the result of the experiments carried out by other scientists could not be explained by this model.
This model fail to explain the location of electrons in the atom.
Question: An element A contains 6 electron in its M shell as valence electrons
(a) Determine the atomic number of A.
(b) identify whether A is a metal or nonmetal.
Answer: (a) As we know that atomic number is numerically equal to the number of electrons
Thus, Atomic no = 16
(b) A is a non metal
Question: Give a suitable reason that why do noble gases have zero valency?
Answer: Noble gases contain maximum number of electrons in their outermost shell and hence they do not have any desire to take part in chemical combination. Thus, they have valency equal to zero and these elements are known as zero valent elements.
Question: Explain why chlorine whether as the element or its compound always has the relative atomic mass of about 35.5.
Answer: The relative atomic mass is the average mass of one of the atom and has to take into account the relative abundances of various isotopes. Natural chlorine always contain about 3/4 × 3517Cl and 1/4 × 3517Cl
Does relative atomic mass of chlorine is 3/4 × 35 + 1/4 × 35 = 35.5
Question: Atomic number could be any accurate parameter in order to identify the element not the mass numbers. Justify the statement.
Answer: In the study of the atomic structure we have seen that the isotopes of an element have different mass numbers whereas in isobars, atoms of variable elements have the same mass number. Hence atomic number of no two elements can be same. Therefore the elements can easily be identified by their atomic number not by their mass numbers.
# Structure of atom Class 9 Science
Do share this post if you liked Structure of Atom Class 9 Notes. For more updates, keep logging on BrainyLads.
How useful was this post?
Click on a star to rate it!
Average rating 5 / 5. Vote count: 1
No votes so far! Be the first to rate this post.
Related Posts

Atoms and Molecules Class 9 Notes | Science | Term 2 |
Work Energy and Power Class 9 | Science | Notes | Term 2 |