Atoms and Molecules Class 9 Notes | Science | Term 2 |
Table of Contents
Atoms and Molecules Class 9 Notes | Science | Term 2 |
# Atoms and Molecules Class 9 Science
Matter is everything around you. Everything that possess mass and occupy space or volume is matter and can be felt by one or more of our five senses.
It was postulated by Maharashi Kanad that if we divide Padarth (matter) we will get smaller and smaller particles. After a time it will not be possible to further divide the matter. The smallest particle was named as Parmanu.
Greek philosopher democratic and Leucippus also stated that if we go on dividing matter stage will come when particle obtained cannot be divided and called these indivisible particles as atoms.
Note: Atom is the smallest particle of an element which may or may not exist freely.
Atoms of Fe, Au , Ag can exist freely whereas atoms of hydrogen and oxygen cannot exist freely.
Molecule is the smallest particle of element or compound that always exist freely.
Law of chemical combination confirmed the idea of atom being the smallest particle of matter.
Law of Conservation of Mass
It was given by lavoisier in 1774. The substance which combined together in a chemical reaction are commonly known as reactants.
The new substance which is formed due to the result of chemical reactions are products.
The law of conservation of mass states that mass can neither be created nor be destroyed in a chemical reaction. It simply means that total mass of product is equal to total mass of reactants.
Law of Constant Proportion
It was given by Proust in 1779.
It is commonly known as law of definite proportion. It states that a chemical compound always consists of same element combined together in the same proportion by mass. In a chemical substance elements are present in definite proportion by mass.
Dalton’s Atomic Theory
- All the matters are made up of a very small particles called as atoms.
- Atom cannot be divided.
- Atom can neither be created nor be destroyed. Only arrangement of atoms occur and no new atom is formed.
- Atoms of different elements have different sizes and masses and also possess different properties.
- The number and kind of atoms in a given compound is fixed.
- During chemical combination atoms of different elements combine in the ratio of small whole number in order to form the compounds.
Drawbacks or Limitations of Dalton’s Atomic Theory
- Atoms of same element can have different masses.
- Atom can be further divided into smaller particles such as electrons, protons and neutrons.
- Atoms of different elements can have same masses.
- Substances made of same kind of atom may have different properties.
Atomic Mass
Atoms are extremely small particles. Hence, the actual mass of atoms are so small that it is difficult to determine the actual masses of individual atoms.
In 1961, IUPAC recommended the use of an isotope of carbon with mass number 12 as the standard reference for measuring atomic masses. It is called carbon 12 and is represented as 12C.
| Atomic Number | Element | Symbol | Atomic Mass |
| 1 | Hydrogen | H | 1 |
| 2 | Helium | He | 4 |
| 3 | Lithium | Li | 7 |
| 4 | Beryllium | Be | 9 |
| 5 | Boron | B | 11 |
| 6 | Carbon | C | 12 |
| 7 | Nitrogen | N | 14 |
| 8 | Oxygen | O | 16 |
| 9 | Fluorine | F | 19 |
| 10 | Neon | Ne | 20 |
| 11 | Sodium | Na | 23 |
| 12 | Magnesium | Mg | 24 |
| 13 | Aluminium | Al | 27 |
| 14 | Silicon | Si | 28 |
| 15 | Phosphorous | P | 31 |
| 16 | Sulphur | S | 32 |
| 17 | Chlorine | Cl | 35.5 |
| 18 | Argon | Ar | 40 |
| 19 | Potassium | K | 39 |
| 20 | Calcium | Ca | 40 |
The atomic mass is now defined as follows:
The atomic mass of an element is simply the relative mass of its atoms that is compared with the mass of an atom of carbon 12 isotope which is taken as 12 units.
Atomic mass unit (amu) may also be defined as 1/12th of the mass of an atom of carbon 12 isotope on the atomic scale.
Gram Atomic Mass
It is the quantity in grams which is numerically equal to the atomic mass of an element on a.m.u scale.
The relation between gram atoms, mass and atomic mass of a substance is
Number of gram atoms = Mass in grams / Gram atomic mass.
How do Atoms Exist?
Atoms of most of the elements are chemically reactive and do not exist in free state or single atom. Atoms usually exist in two ways:
- In the form of molecules
- In the form of ions
Molecules
A molecule is the group of two or more atoms which are held strongly bi some kind of attractive forces. Such an attractive force holding the atoms together is called chemical bond.
Molecule can simply be defined as the smallest particle of element or compound which can exist freely under ordinary conditions and show all the properties of that substance.
Ions
An ion may be defined as atom or group of atom having positive or negative charge. The ion having one or more positive charge is commonly known as cation whereas the ion having one or more negative charges is known as anions.
Atomicity
The number of atoms present in one molecule of an element is called its atomicity.
In H2 shows the atomicity of hydrogen is 2.
Molecular Formula of Compound
The symbolic representation of molecule of a compound is generally known as molecular formula.
The ratio of number of atoms in the molecule of a compound can easily be calculated by knowing the ratio of their masses and atomic masses of elements constituting the molecule of the compound.
By knowing the chemical formula of a substance following information could be gathered:
- Name of the substance
- Name of various elements present in that substance
- Chemical formula of substance represent one molecule of that substance.
- Relative mass of various element in the compound.
- It represent one mole of the substance
- The gram molecular mass of that substance can effectively be calculated.
Valency of an Element
It is generally defined as the combining capacity of an element and is equal to the number of hydrogen atom on number of chlorine atom or double the number of oxygen atom with which one atom of the element combines.
Valencies of Positive Ions
It depends upon the charge of present on the ion. The valencies of some of the positive ions are listed below:
| Monovalent | Bivalent | Trivalent |
| Hydrogen H+ | Calcium Ca2+ | Aluminium Al3+ |
| Potassium K+ | Magnesium Mg2+ | Chromium Cr3+ |
| Sodium Na+ | Copper Cu2+ | Iron Fe3+ |
| Silver Ag+ | Cobalt Co2+ | Gold Au3+ |
Valencies of Negative Ions
| Monovalent | Bivalent | Trivalent |
| Chloride Cl¯ | Sulphide S2- | Nitride N3- |
| Bromide Br¯ | Oxide O2- | Phosphate (PO4)3- |
| Hydroxide (OH)¯ | Carbonate (CO3) 2- | Phosphide P3- |
| Nitrate (NO3) ¯ | Sulphate (SO4) 2- | Borate (BO3)3- |
| Nitrite (NO2) ¯ | Sulphite (SO3) 2- | Arsenate (AsO3)3- |
Formulae of Molecular Compounds
While writing the chemical formula of the given molecular compound, we have to write the constituent elements of the compound and then there valencies then we cross over the valency is of the combining atoms.
Example:
Formula of hydrogen Chloride – HCl
| Symbol | H | Cl |
| Valency | 1 | 1 |
Some Important Molecular Formulae:
| Compound | Molecular Formula |
| Nitric acid | HNO3 |
| Sodium hydroxide | NaOH |
| Sodium Oxide | Na2O |
| Aluminium Carbonate | Al2O3 |
| Zinc Sulphide | ZnS |
| Potassium Nitrate | KNO3 |
| Calcium Chloride | CaCl2 |
| Aluminium Sulphate | Al2(SO4)3 |
| Zinc Oxide | ZnO |
Molecular Mass
Molecular mass of a substance that is an element or a compound is the average relative mass of its molecules as compared with that of an atom of carbon-12 isotope taken as 12.
Calculation of molecular Mass:
Formula mass of Calcium Oxide (CaO) = Atomic mass of Calcium + Atomic mass of Oxygen
= 40 + 16 = 56 u
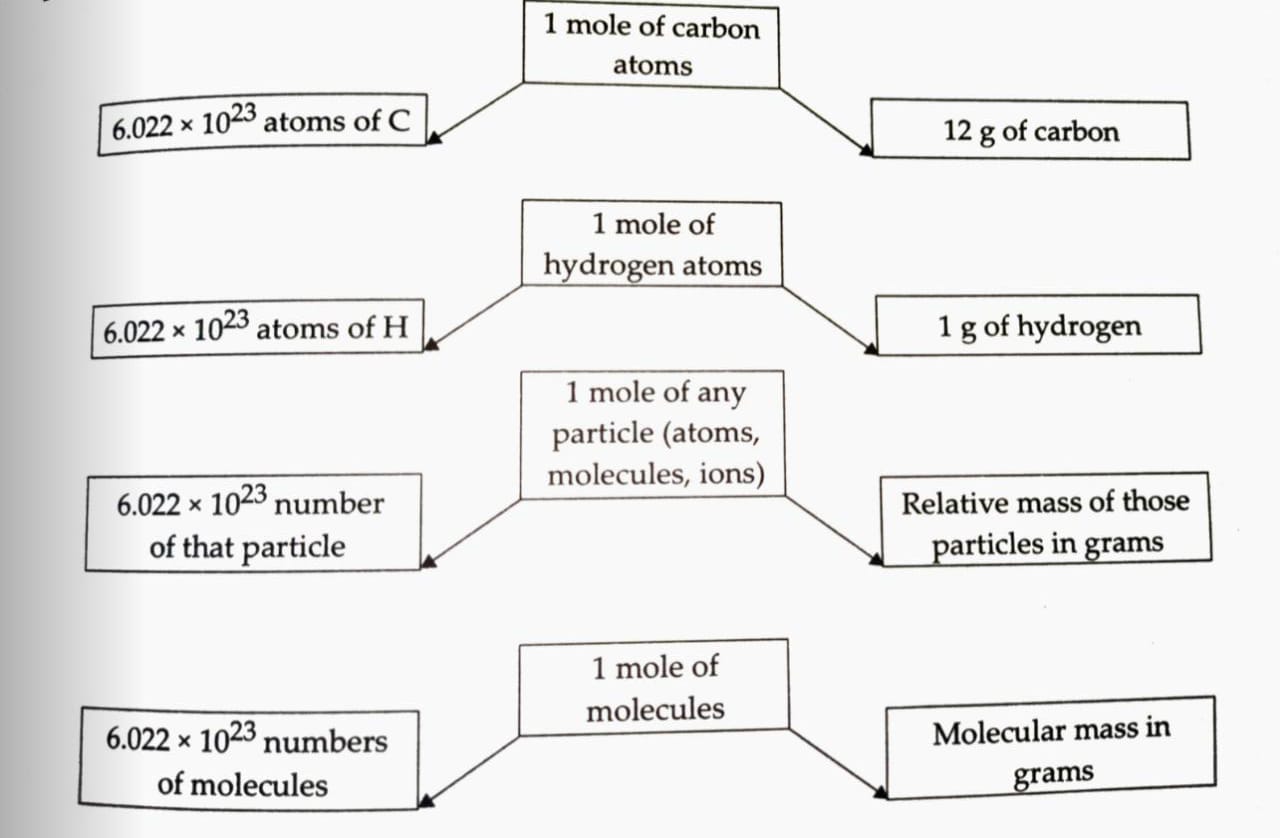
Mole Concept
A mole of atom is defined as that amount of the substance (Element) which has mass equal to gram atomic mass of the element.
Number of Moles = Given mass / Molar Mass = Given no of molecules / Avogadro No
Question: Calculate the mass of single atom of sulphur?
Answer: Gram atomic mass of Sulphur= 32 g
Mass of one sulphur atom = Gram atomic mass / 6.022 × 1023
Mass of one sulphur atom= 32 / 6.022 × 1023 = 5.31 × 10-23 g
Question: Calculate the molecular mass of C12H22O11 .
Answer: Molecular mass of C12H22O11 = 12 x At. mass of C +22 x At. mass of H + 11 x At. mass of O
Molecular mass of C12H22O11 = ( 12 X 12 + 22 X 1 + 11 X 16) u = (142 + 22 + 176) u = 342 u
Question: How many molecules are there in 0.5 mol of water?
Answer: 1 mol of water contain 6.022 × 1023 molecules.
0.5 mol of water contain 3.011 × 1023 molecules.
Question: If 100g of pure water taken from different sources is decomposed by passing electricity through it then 11 grams of hydrogen and 89 grams oxygen are always obtained. Identify the chemical law that is illustrated by the statement?
Answer: As mass of reactants is equal to sum of mass of products. Thus law of conservation of mass is followed as per the given statement.
Question: Why is the statement ‘1 mole of Hydrogen ‘ not accurate? What should be the correct statement?
Answer: 1 mole of hydrogen does not tell whether we have one mole of hydrogen atoms or one mole of hydrogen molecules. The correct statement should be one mole of hydrogen atoms or one mole of hydrogen molecules.
Question: Which among the following has greater number of atoms 11.5 g of sodium or 15 g of calcium ?
Answer: No of moles of Sodium = Mass of sodium / Gram atomic mass = 11.5 / 23 = 0.5 moles
No of moles of Calcium = Mass of Calcium / Gram atomic mass = 15 / 40 = 0.375 moles
Sodium has more number of moles as compared to calcium. Thus, it will have more number of atoms than calcium.
# Atoms and Molecules Class 9 Science
# Atoms and Molecules Class 9 Science NCERT Solutions
Do share this post if you liked Atoms and Molecules Class 9 Notes. For more updates, keep logging on BrainyLads.