Aldehydes and Ketones Class 12 | Carboxylic Acids | Chapter 12 |
Table of Contents
Aldehydes and Ketones Class 12 | Carboxylic Acids | Chapter 12 | Chemistry |
Aldehydes, Ketones and Carboxylic Acids
All these organic compounds contain carbon – oxygen double bond, i.e., >C=O called the carbonyl group.
Nomenclature of Aldehydes
| Formula | Common Name | IUPAC Name |
| HCHO | Formaldehyde | Methanal |
| CH3CHO | Acetaldehyde | Ethanal |
| CH3CH2CHO | Propionaldehyde | Propanal |
| CH3CH2CH2CHO | Butyraldehyde | Butanal |
| Isobutyraldehyde | 2-Methylpropanal | |
| Phenylacetaldehyde | 2-Phenylethanal | |
| CH2=CHCHO | Acrolein | Prop-2-enal |
| CH3CH=CHCHO | Crotonaldehyde | But-2-en-1-al |
| C6H5CH=CHCHO | Cinnamaldehyde | 3-Phenylprop-2-en-1-al |
 |
Benzaldehyde | Benzaldehyde |
 |
Phthaldehyde | Benzene-1,2-dicarbaldehyde |
 |
m-Bromobenzaldehyde | 3-Bromobenzaldehyde |
Nomenclature of Ketones
| Formula | Common Name | IUPAC Name |
 |
Acetone | Propanone |
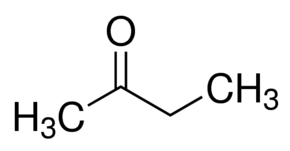 |
Ethyl methyl ketone | Butan-2-one |
 |
Methyl n-propyl ketone | Pentan-2-one |
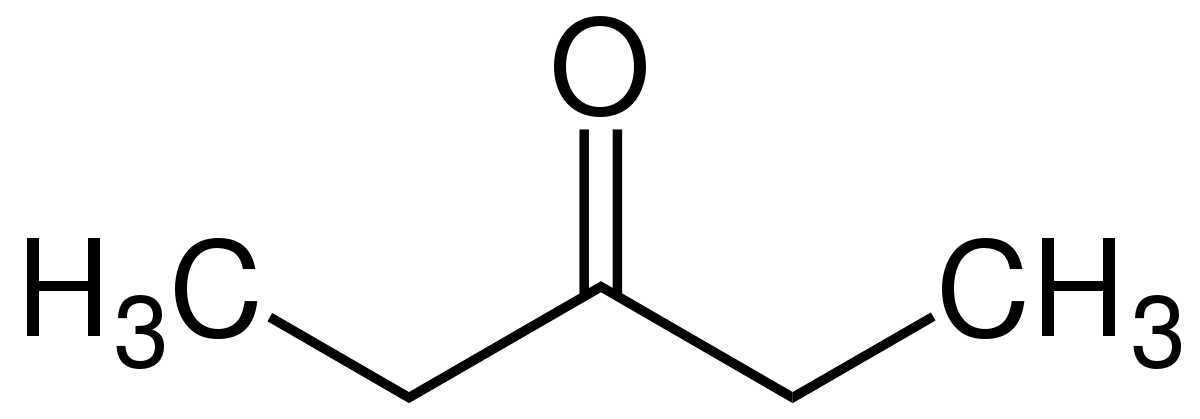 |
Diethyl ketone | Pentan-3-one |
 |
Di-isopropyl ketone | 2,4-Dimethylpentan-3-one |
 |
Mesityl oxide | 4-Methylpent-3-en-2-one |
 |
β-Methylcyclopentanone | 3-Methylcyclopentanone |
 |
α-Methylcyclohexanone | 2-Methylcyclohexanone |
 |
Methyl phenyl ketone | 1-Phenylethan-1-one |
Nomenclature of Carboxylic Acids
| Formula | Common Name | IUPAC Name |
| HCOOH | Formic Acid | Methanoic Acid |
| CH3COOH | Acetic Acid | Ethanoic Acid |
| CH3CH2COOH | Propionic Acid | Propanoic Acid |
| CH3CH2CH2COOH | Butyric Acid | Butanoic Acid |
| Lactic Acid | 2-Hydroxypropanoic Acid | |
| HOOC–COOH | Oxalic Acid | Ethanedioic Acid |
| HOOC-CH2-COOH | Malonic Acid | Propanedioic Acid |
| HOOC-(CH2)2-COOH | Succinic Acid | Butanedioic Acid |
| HOOC-(CH2)3-COOH | Glutaric Acid | Pentanedioic Acid |
| HOOC-(CH2)4-COOH | Adipic Acid | Hexanedioic Acid |
 |
Benzoic Acid | Benzenecarboxylic Acid |
 |
Phenylacetic Acid | 2-Phenylethanoic Acid |
 |
Phthalic Acid | Benzene-1,2-dicarboxylic Acid |
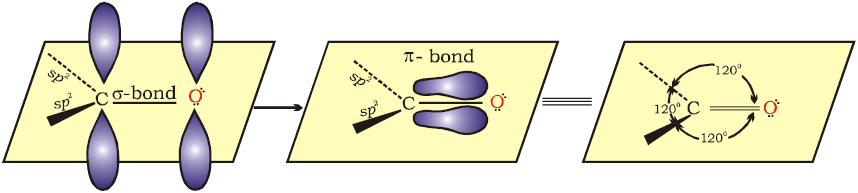
Structure of Carbonyl Group
- In the formation of carbonyl group , C uses sp2-hybrid orbitals while O uses its unhybrid atomic orbital.
- The carbonyl Carbon and three atoms attached to it lie in same plane and the π electron cloud is above and below this plane .
- The oxygen atom in carbonyl group is more electronegative than carbon atom . As a result , carbon – oxygen bond is polarised
- Carbonyl Carbon acquires a small positive charge and thus acts as an electrophile (Lewis Acid) .
- Carbonyl Oxygen carries a small negative charge and hance behave as nucleophile (Lewis Base).
- Thus , Carbonyl group is polar in nature and Thus Aldehydes and ketones have large dipole moments.
.
Common Methods for Preparation of Both Aldehydes and Ketones
1) From Alcohols
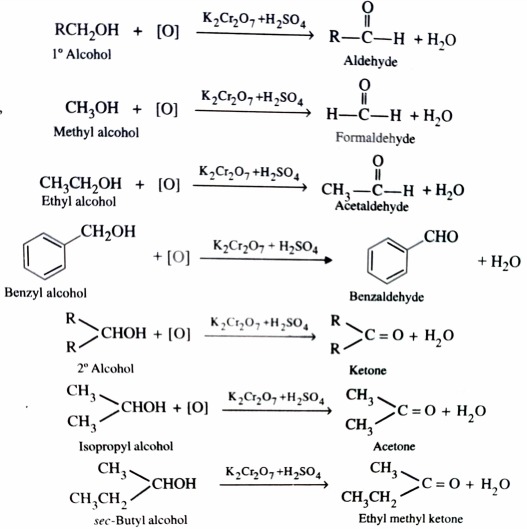
a)Oxidation with Chromium and Manganese Reagents
Alcohols on controlled oxidation give aldehyde or ketone. Whereas Primary alcohol give aldehydes , secondary alcohols give ketones . The common oxidising agent used are acidified K2Cr2O7 , aqueous or alkaline KMnO4 and chromic anhydride.
The aldehyde formed in this process are readily oxidised to give carboxylic acids if allowed to remain in the reaction mixture .
Therefore , to prevent further oxidation of aldehydes to carboxylic acids , the aldehydes are distilled off as soon as they are formed .
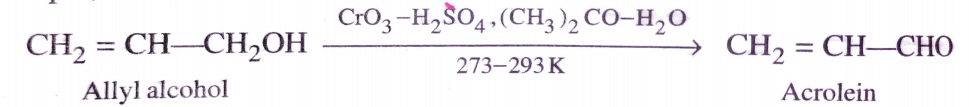
b)Jone’s Reagent
It is a solution of CrO3 in H2SO4 in aqueous acetone . It has also been used to oxidise 1º alcohols to aldehydes at room temperature or below (273-293K) . However , if excess of reagent is used , aldehydes are further oxidised to carboxylic acids . This reagent is very selective and does not attack double or triple bonds . It has also been used to oxidise allylic and benzylic 1º and 2º alcohols.
c)Corey’s Reagent or Pyridinium Chlorochromate (PCC)
Its main advantage is that oxidation stops at the aldehyde stage thereby preventing the further oxidation of aldehydes to carboxylic acids.
Further , this reagent do not attack the double bonds which remain unaffected during these oxidation reactions
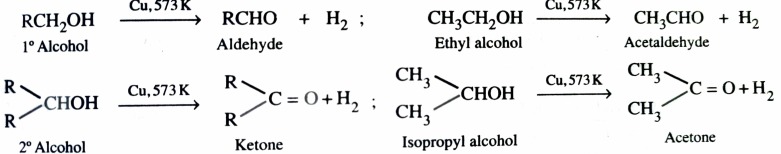
2) Catalytic Dehydrogenation of Alcohols
The dehydrogenation of alcohols is carried out by passing the vapours of alcohol over reduced copper at 573K . Primary alcohols give aldehyde while secondary alcohol give ketones .
3) From Hydrocarbons
a)By Ozonolysis of Alkenes
Alkene react with ozone to form ozonides which upon subsequent reductive cleavage with Zn dust and water gives aldehyde and ketones . Under these conditions , ethylene gives formaldehyde , symmetrically disubstitued alkenes give other aldehyde whilw tetrasubstitued alkenes gives ketones.
b)Hydration of Alkynes
In presence of hot dil.H2SO4 and HgSO4 , alkynes add a molecule of water to form aldehydes or ketones.
Note : Formaldehyde cannot be prepared by this method
c)Hydroboration Oxidation of Alkynes
Aldehydes and Ketones can also be prepared by hydroboration oxidation of alkynes . Whereas symmetrical non-terminal alkynes give a single ketone , unsymmetrical non-terminal alkynes give a mixture of both possible ketones in which methyl ketone predominates.
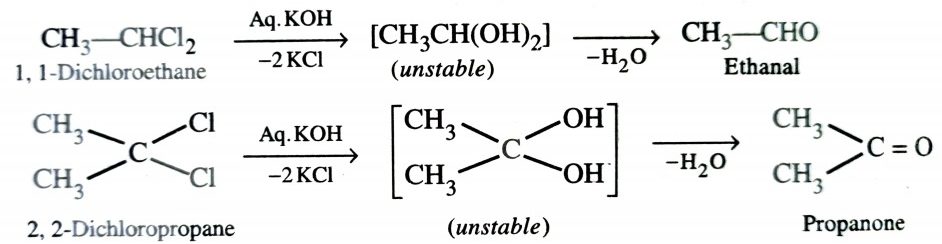
d)From Gem-Dihaldide
Gem-Dihalides (Halides having two halogens on same carbon) upon alkaline hydrolysis give the corresponding aldehydes or ketones.
4) From Carboxylic Acids
a)By Dry Distillation of Calcium Salts of Fatty Acids
When calcium salt of fatty acid is distilled with calcium formate , an aldehyde is produced . Distillation of Calcium formate alone gives formaldehyde while that of calcium salt of any other acid gives a symmetrical ketone .
However , this method is not suitable for preparation of aldehydes except formaldehyde since the yields are low .
b)By Passing the Vapours of Fatty Acid over Manganous Oxide at 573K
Formic Acid alone gives formaldehyde . Acids other than formic acid give ketones whereas a mixture of formic acid and any other acid gives aldehyde other than formaldehyde.
Preparation of Aldehydes Only
1)From Acid Chlorides by Rosenmund Reduction
Catalytic reduction of acid chlorides to the corresponding aldehydes is called Rosenmund reduction . This reaction is carried out by passing hydrogen gas through boiling xylene solution of the acid chloride in presence of Pd catalyst supported over BaSO4 (partially poisoned by addition of sulphur).
Note : Formaldehyde cannot be prepared by this method since formyl Chloride , HCOCl is unstable at room temperature.
2)From Nitriles by Stephen Reduction
The partial reduction of alkyl or aryl cyanide to the corresponding aldehydes with a suspension of anhydrous stannous chloride in dry ether saturated with hydrogen chloride at room temperature followed by hydrolysis is called Stephen reduction . During this reaction , imine hydrochloride first gets precipitated which on hydrolysis with boiling water gives the corresponding aldehyde.
Alternatively , Nitriles can be selectively reduced by diisobutylaluminium hydride (DIBAL-H) to imines which upon hydrolysis give aldehydes.
Similarily , esters can also be reduced to aldehydes by DIBAL-H . This reaction is carried out at low temperature.
Preparation of Aromatic Aldehyde only
By oxidation of Methyl Benzene
Strong oxidising agent such as acidified or alkaline Potassium permanganate oxidise Toluine and its derivatives to benzoic acid .
However , it is possible to stop the reaction at aldehyde stage with suitable reagents which convert methyl group to an intermediate which is difficult to oxidise further .
i)Use of Chromyl Chloride (CrO2Cl2)
Toluene can be oxidised to benzaldehyde with a solution of chromyl chloride in CS2 or CCl4 .The brown chromium complex thus precipitated is separated and decomposed with dilute acids to give benzaldehyde . This reaction is called Etard Reaction .
ii)Use of Chromic Oxide (CrO3)
Aromatic aldehyde can also be prepared by oxidation of toluene and its derivatives with Chromium trioxide in acetic anhydride . The gem-diacetate first formed is isolated and then hydrolysed with alkali to yield the corresponding aromatic aldehyde .
iii)By Side Chain Halogenation Followed by Hydrolysis
Side chain chlorination of toluene gives benzal chloride which upon hydrolysis gives benzaldehyde .
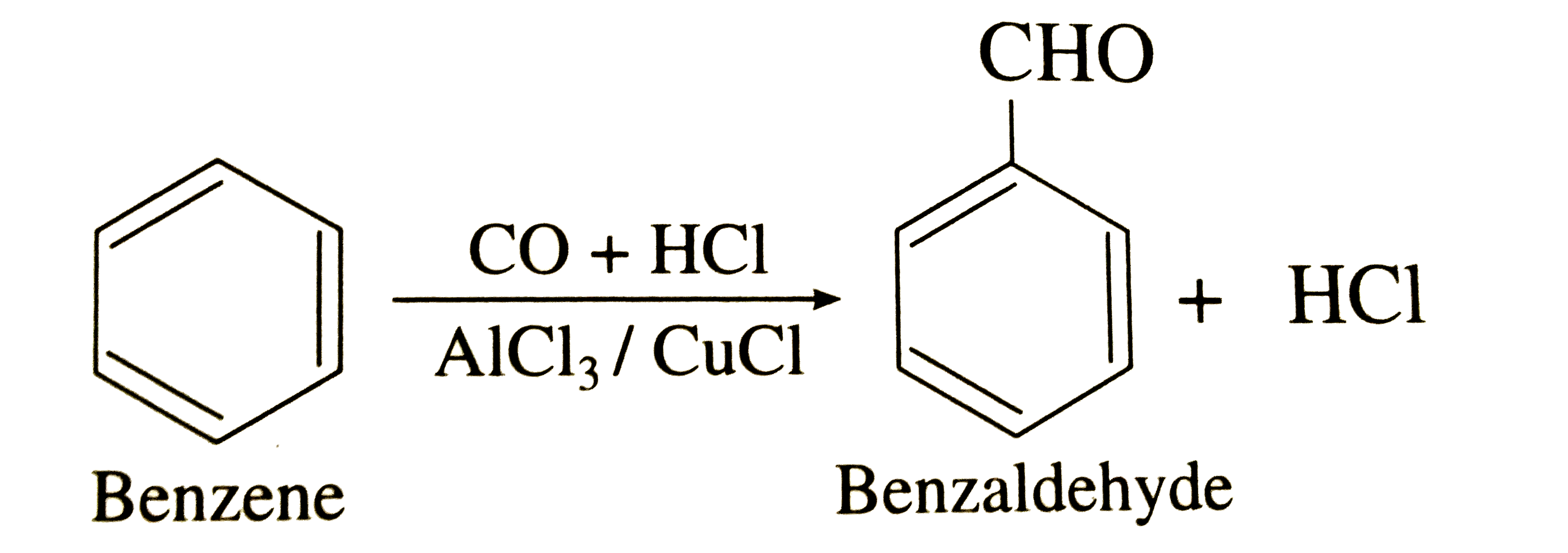
iv)By Gattermann Koch Reaction
When a mixture of CO and HCl gas is passed through benzene at 323 K in presence of a catalyst consisting of anhydrous aluminium chloride and a small amount of CuCl , benzaldehyde is formed. This reaction is called Gattermann formylation or Gattermann Koch Reaction .
Preparation of Ketones Only
1) From Acid Chlorides
Ketones can be easily prepared by the action of suitable dialkylcadmium ( prepared by the reaction of cadmium chloride with Grignard reagent ) on an acid chloride .
Grignard Reagent cannot be directly used in the reaction because Grignard reagent being more reactive than dialkylcadmium reagents would react with the ketones formed in the reaction to give ultimately 3º alcohols .
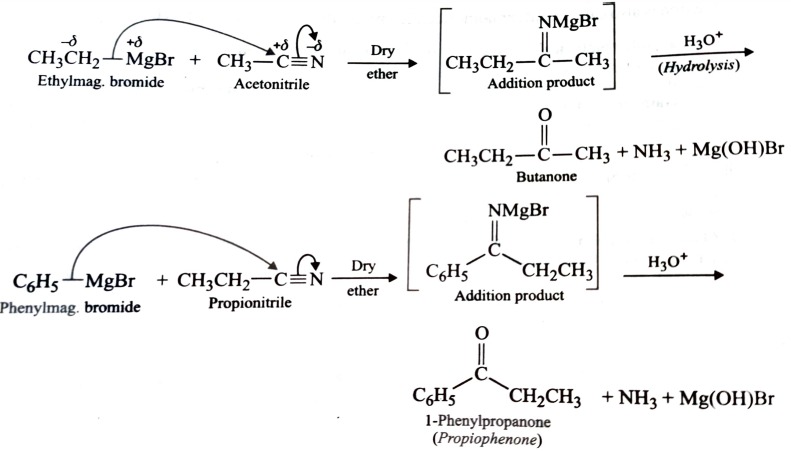
2) From Nitriles
Both Aliphatic and Aromatic ketones can be prepared by the action of a suitable Grignard reagent on an alkyl or aryl nitrile followed by acid hydrolysis of the intermediate addition product .
3)From Benzene or Substituted Benzene by Friedel Crafts Acylation
This is one of the most convenient and widely used method for the preparation of of aromatic ketones in which the ketonic group is directly attached to atleast one aromatic ring . It involves the treatment of an aromatic hydrocarbon with an acid halide in presence of a Lewis acid such as anhydrous aluminium chloride .
Related
Physical Properties of Aldehydes and Ketones
1)Boiling Point
- The boiling point of aldehyde and ketone are higher than hydrocarbons and ethers of comparable molecular mass due to weak intermolecular association in aldehyde and ketone arising out of dipole dipole interaction .
- Their boiling point are lower than those of alcohol of similar molecular mass due to absence of intermolecular hydrogen boding.
2)Solubility
- Lower aldehydes and ketones containing upto four carbon atoms such as methanal , ethanal , propanone etc are soluble in water due to H bonding between the polar carbonyl group and the water molecules.
- As the size of alkyl group increases with increasing molecular mass , its solubility decreases.
- All aldehydes and ketones are fairly soluble in organic solvents .
3)Smell
- The lower aldehydes have shap pungent (unpleasant ) odour.
- As the size of molecule increases , odour become less pungent and more fragrant .
- Lower ketones are generally pleasant smelling liquids while higher ones are solids.
- Many naturally occuring aromatic aldehydes and ketones have been used in blending of perfumes and flavouring agents .
Chemical Properties of Aldehydes and Ketones
- Nucleophilic Addition Reactions
Alkenes , aldehydes and ketones are unsaturated compounds . Unlike alkenes which show electrophilic addition reactions , aldehydes and ketones undergo nucleophilic addition reactions.
Mechanism of Nucleophilic Addition Reaction
- Due to greater electronegativity of Oxygen as compared to carbon , carbon atom of carbonyl group carries a small positive charge and hence behave as electrophile .
- A nucleophile readily attacks the electrophile carbon atom of polar carbonyl group from a direction perpendicular to plane of sp– hybridized orbital of carbonyl carbon.
- During this process , complete transfer of π electrons of the carbon oxygen double bond takes place from carbon to oxygen atom , hybridization of carbon changes from sp2 to sp3 and a tetrahedral alkoxide intermediate is formed .
- This intermediate then picks up a proton to give electrically neutral addition product .
Reactivity
As the number and the size of alkyl group increases , the attack of the nucleophile on carbonyl group becomes more and more difficult due to steric hindrance . In other words , as crowding increases reactivity decreases.
Thus aldehydes are more reactive than ketones towards nucleophilic addition reaction .
Addition of Hydrogen Cyanide (HCN)
Both aldehydes and ketones add a molecule of hydrogen cyanide to form cyanohydrin.
With pure HCN , reaction occurs very slowly so it is catalysed by a base . The CN- thus being a strong nucleophile easily adds to carbonyl compound .
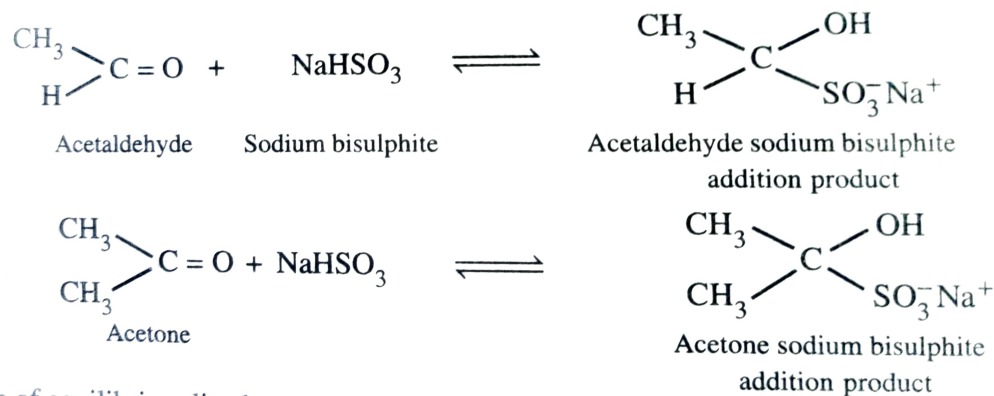
Addition of Sodium Hydrogensulphite
Most of the aldehyde and aliphatic methyl ketone when treated with saturated sodium bisulphite solution add a molecule of sodium bisulphite to form bisulphite addition product.
This reaction is used in purification and separation of aldehyde and ketone from non-carbonyl compounds .
The bisulphite addition product are colourless crystalline solids.
Addition of Grignard Reagent
Aldehyde and ketone add on grignard reagent to form addition products which upon hydrolysis with water or dilute mineral acids give alcohol.
In these reaction , the alkyl or aryl group of the grignard reagent is transferred to the carbon atom of the carbonyl group .
The type of alcohol formed depend upon the aldehyde or ketone used.
Addition of Alcohols
Addition of one molecule of monohydric alcohol to an aldehyde in presence of dry HCl gas yields α-Alkoxyalcohols called hemiacetal .
These hemiacetal then react with one more molecule of alcohol to give gem-dialkoxy compounds known as acetals.
Dry hydrogen chloride protonates the oxygen of carbonyl compound thereby increasing the electrophilicity of carbonyl carbon and hence facilitating nucleophilic attack by alcohol molecule.
Addition of Ammonia Derivatives
It is carried out in weakly acidic medium to form compound containing C=N group . It proceeds with loss of water molecule. The reaction is reversible and is catalysed by acids . The equilibrium favours ythe product formation due to rapid dehydration of tetrahedral addition product.
- Reaction with Hydroxylamine : Aldehyde and ketone react with hydroxylamine to form oximes .
- Reaction with Hydrazine : Aldehydes and ketones react with hydrazines to form Hydrazones .
- Reaction with Phenylhydrazine : Aldehydes and ketones react with phenylhydrazine to form Phenylhydrazones.
- Reaction with 2,4-dinitrophenylhydrazine (DNP) : Aldehyde and Ketone react with 2,4-dinitrophenylhydrazine to form 2,4-dinitrophenylhydrazones.
- Reaction with semicarbazide : Both aldehyde and ketone react with semicarbazide to form semicarbazones .
All the above product formed are well defined crystalline solids with sharp melting points . Therefore , they are used for characterization and identification of aldehydes and ketones.
2) Reduction
Reduction to Alcohols
Aldehydes and Ketones on reduction give primary and secondary alcohol . Reduction is carried out either catalytically with Hydrogen in presence of Ni, Pt or Pd or chemically with lithium aluminium hydride or Sodium borohydride.
Reduction to Hydrocarbon
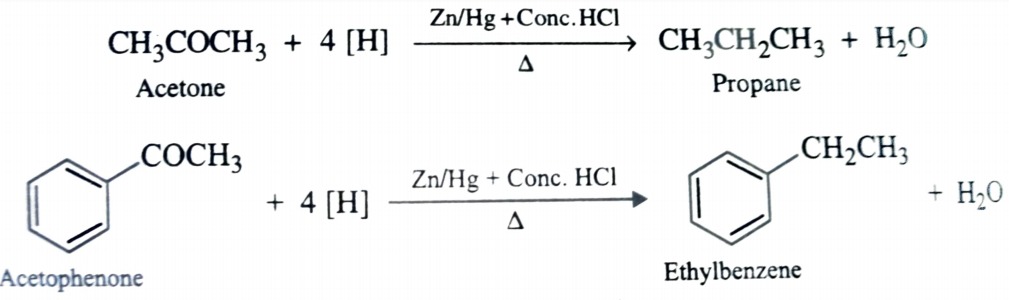
- Clemmensen reduction : The reduction of aldehyde and ketone to corresponding hydrocarbon with amalgamated zinc and concentrated hydrochloric acid is called Clemmensen reduction . This reaction works better with ketone than aldehyde .
- Wolff-Kishnner Reduction : The reduction of aldehydes and ketones to corresponding hydrocarbons by heating them with hydrazine and KOH or potassium tert-butoxide in a high boiling solvent such as ethylene glycol is called Wolff – Kishner reduction .
- With HI + Red Phosphorous : On heating an aldehyde or ketone with hydriodic acid and red phosphorous to 423 K , it is reduced to corresponding alkane .
3) Oxidation
Aldehydes are easily oxidised to carboxylic acids containing the same number of carbon atoms . The reason for this easy oxidation is due to the presence of a hydrogen atom on the carboxyl group which can be converted into OH group without involving cleavage of any other bond . Hence , they are oxidised even by weak oxidizing agents . As a result , aldehydes are strong reducing agents .
Tollen’s Reagent
Tollen’s reagent is an ammonical solution of silver nitrate . When an aldehyde is heated with Tollen’s reagent , the latter is reduced to metallic silver which deposits on the walls of the test tube as bright silver mirror . Since , ketone does not show this reaction thus ; it is used as test to distinguish ketones and aldehydes . The silver thus deposits shine like a mirror . Hence this test is known as silver mirror test . Both aliphatic and aromatic aldehyde reduces Tollen’s reagent .
RCHO (Aldehyde) + 2 [Ag(NH3)2] (Tollen’s reagent) +3OH– → RCOO– (Carboxylate ion) + 2Ag ↓ (Silver mirror) +4NH3 + 2H2O
Fehling’s Solution
Fehling’s solution is an alkaline solution of CuSO4 containing some Rochelle salt, i.e., sodium potassium tartrate and is prepared by adding alkaline solution of Rochelle salt (called Fehling solution B ) to an aqueous solution of CuSO4 (called Fehling solution A) . When an aliphatic aldehyde is heated with Fehling ‘s solution , the latter is reduced to give a red ppt. of cuprous oxide .
Aromatic aldehyde and ketone do not give this test .
RCHO (Aldehyde) + 2 Cu2+ + 5 OH– → RCOO– (Carboxylate ion) + Cu2O ↓ (cuprous oxide)(red ppt.) + 2H2O
Haloform Reaction
Aldehydes and ketones containing CH3CO- group , on treatment with excess of halogen in presence of alkali produce a haloform . In this reaction , all the three H atom of methyl group are first replaced by halogen atom to form trihaloaldehyde or trihaloketone which reacts with alkali to yield a haloform and salt of carboxylic acid containing one carbon atom less than staring aldehyde / ketone .
CH3CHO (Acetaldehyde) + 3I2 CI3CHO (Triiodoacetaldehyde) + 3HI
CI3CHO + NaOH CHI3 (Iodoform) (Yellow ppt) + CH3COONa
4) Reaction Due to α-Hydrogen
The acidity of α-hydrogens is partialy due to -I effect of carbonyl group .
Aldol Condensation
Two molecules of Aldehyde or ketones containing α – hydrogen atoms on treatment with dilute base undergo condensation to form β-hydroxyaldehydes or β-hydroxyketones . This reaction is called aldol condensation . The product of aldol condensation when heated with dilute acids undergo dehydration leading to formation of α,β- unsaturated aldehydes or ketones .
Cross Aldol Condensation
Aldol condensation between two different aldehydes or two ketones or between one aldehyde and one ketone is called cross aldol condensation . If both of them contain α-hydrogens , it gives a mixture of four products.

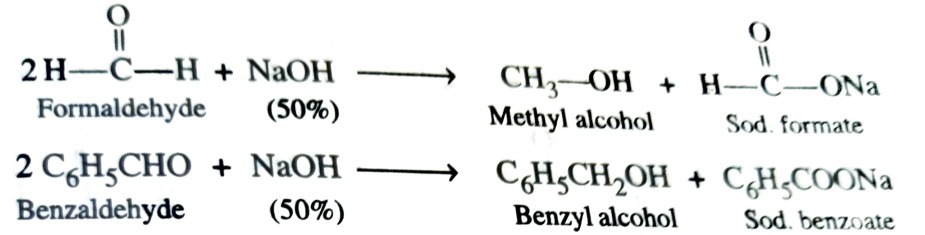
5) Cannizzaro Reaction
Aldehydes which do not contain an α-hydrogen atom , when treated with concentrated alkali solution undergo disproportionation , i.e., self oxidation reaction . As a result , one molecule of aldehyde is reduced to corresponding alcohol at the cost of other which is oxidised to corresponding carboxylic acid . This reaction is called Cannizzaro reaction.
6) Electrophilic Substitution Reaction
Aldehydic group and ketonic group are electron withdrawing , therefore , they are deactivating and m-directing .
Halogenation
It is difficult to carry out both in aliphatic and aromatic aldehydes and ketones since chain halogenation occurs very fast .
However , when acetophenone is treated with Bromine in presence of excess of anhydrous aluminium chloride , m-bromoacetophenone is formed .
Nitration
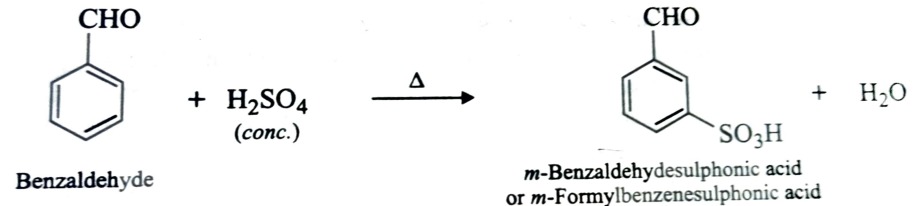
Sulphonation
Methods of Preparation of Carboxylic Acids
1)From Primary Alcohols and Aldehydes
The primary alcohols and aldehydes are readily oxidised to corresponding carboxylic acids with common oxidising agent such as KMnO4
2)From Alkylbenzenes
Due to higher reactivity of the benzylic hydrogens as compared to other hydrogens on alkyl chain , oxidation occurs at this carbon . During these oxidations , the aromatic nucleus remain intact but the entire side chain is oxidised to a -COOH group irrespective of the length of the carbon chain .
3)From Nitriles and Amides
Nitriles are first hydrolysed to amides and then to acids in the presence of boiling mineral acids or alkalies as catalyst .
4)From Grignard Reagents
Carboxylic acid can also be prepared by action of Grignard reagents on carbon dioxide . The addition product so formed on acidification with mineral acid gives the carboxylic acids.
5)From Acid Halides or Anhydrides
Acid chlorides are hydrolysed by water to give carboxylic acids . Anhydrides on the other hand , are slowly hydrolysed by cold water and rapidly by hot water to give corresponding acids.
Physical Properties of Carboxylic Acids
Solubility
- Simple alphatic carboxylic acids are miscible in water due to formation of hydrogen bonds with water .
- All carboxylic acids are soluble in less polar organic solvents such as benzene , ether and alcohol .
Boiling Points
- The boiling point of carboxylic acid are even much higher than those of hydrocarbon and somewhat higher than those of aldehydes , ketones and even alcohols of comparable molecular masses .
- This is due to more extensive association of carboxylic acid molecules through intermolecular hydrogen bonding.
Chemical Properties of Carboxylic Acids
Reaction Involving Cleavage of O-H Bond
- Ka called dissociation constant of the acid . Greater the value of Ka , greater is the tendency of acid to ionize and hence stronger is the acid .
- Carboxylic acids are weaker than mineral acids , but they are stronger than alcohols and many simple phenols . This is due to the reason both carboxylic acid and carboxylate anion are stabilized by resonance but neither the alcohols nor their alkoxide ions are stabilized by resonance .
- Electron withdrawing group increases the acidity of carboxylic acids by stabilising the conjugate base through delocalisation of negative charge by inductive or resonance effect .
- Electron donating group decreases the acidity by destabilising the conjugate base .
- Direct attachment of phenyl or vinyl group increases the acidity of corresponding carboxylic acids.
Reaction with Metals
2RCOOH + 2Na → 2RCOONa + H2
2CH3COOH + 2Na → 2CH3COONa (Sodium acetate) + H2
Reaction with Alkalies
RCOOH + NaOH → RCOONa (Sod. Carboxylate) + H2O
Reaction Involving Cleavage of C-OH Bond
Esterification
When carboxylic acids are heated with alcohols or phenols in presence of conc . H2SO4 or dry HCl gas , esters are formed .
RCOOH + R’OH → RCOOR’ + H2O
Formation of Acid Chlorides
Carboxylic acid react with thionyl chloride , phosphorous pentachloride or phosphorous trichloride to form acid chloride by replacement of OH group by Cl atom .
Reaction with Ammonia
When treated with ammonia , carboxylic acids form ammonium salts which upon heating loose a molecule of water to form acid amides .
Reaction Involving -COOH Group
Reduction to Alcohols
The reduction of carboxylic acid with lithium aluminium hydride gives primary alcohol . During this reduction , CO group of carboxyl group is reduced to CH2 .
Decarboxylation
Sodium salt of Carboxylic acid when heated with soda lime undergo decarboxylation to yield alkanes .
Hundsdiecker Reaction
The decomposition of silver salt of a carboxylic acid with Bromine in refluxing CCl4 to form an alkyl or aryl bromide with one carbon less than original acid .
Substitution Reaction in Hydrocarbon Part
Hell – Volhard Zelinsky Reaction
The reaction of an aliphatic carboxylic acid containing α- hydrogens with Cl2 or Br2 in presence of a small amount of red phosphorous to give α – haloacids is called Hell – Volhard – Zelinsky reaction .
Ring Substitution
As the -COOH group is electron withdrawing therefore , it is meta directing . In other words , Electrophilic substitution in benzoic acid occurs at meta position .
Do share the post if you liked Aldehydes and Ketones Class 12. For more updates, keep logging on BrainyLads