Haloalkanes and Haloarenes Class 12 Notes | Chapter 10 | Chemistry |
Table of Contents
Haloalkanes and Haloarenes Class 12 Notes | Chapter 10 | Chemistry | CBSE |
Haloalkanes and Haloarenes Class 12 Notes
Haloalkanes and Haloarenes Class 12 Notes : Haloalkane and Haloarene are obtained by the replacement of a hydrogen atom of an alkane and arene respectively by a halogen atom (F , Cl , Br , I ). Haloalkane contain halogen atom attached to the sp3 hybridised carbon atom of an alkyl group whereas haloarenes contain halogen atom attached to sp2 hybridised carbon atoms of an aryl group.
Nomenclature of Haloalkanes
| Structural Formula | Common Name | IUPAC Name |
| CH3 — Cl | Methyl chloride | Chloromethane |
| CH3 — CH2—Br | Ethyl bromide | Bromoethane |
| CH3 — CH2— CH2— F | n-Propyl fluoride | 1-Fluoropropane |
 |
Isopropyl iodide | 2-Iodopropane |
| CH3 — CH2— CH2—CH2—Cl | n-Butyl chloride | 1-Chlorobutane |
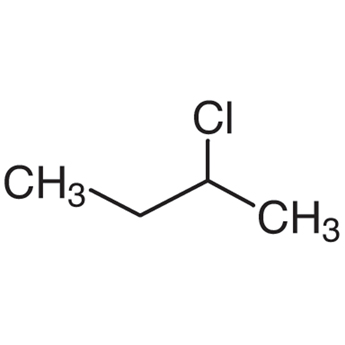 |
sec-Butyl chloride | 2-Chlorobutane |
 |
Isobutyl chloride | 1-Chloro-2-methylpropane |
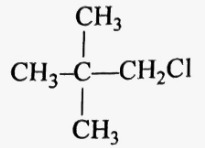
 |
tert-Butyl chloride | 2-Chloro-2-methylpropane |
| CH3CH2CH2CH2CH2Cl | n-Pentyl chloride | 1-Chloropentane |
 |
Isopentyl chloride | 1-Chloro-3-methylbutane |
 |
tert-Pentyl chloride | 2-Chloro-2-methylbutane |
 |
Neopentyl chloride | 1-Chloro-2,2-dimethylpropane |
| CH2Cl2 | Methylene dichloride | Dichloromethane |
| CH3CHBr2 | Ethylidene dibromide | 1,1-Dibromoethane |
| CH3 —CCl2 —CH3 | Isopropylidene dichloride | 2,2-Dichloropropane |
| CH3CH2CHCl2 | Propylidene dichloride | 1,1-Dichloropropane |
| BrCH2CH2CH2Br | Trimethylene dibromide | 1,3-Dibromopropane |
| CHCl3 | Chloroform | Trichloromethane |
| CCl4 | Carbon tetrachloride | Tetrachloromethane |
Nomenclature of Haloarenes (Aryl Halides)
| Structural Formula | Common Name | IUPAC Name |
 |
Chlorobenzene | Chlorobenzene |
 |
o-Chlorotoluene | 1-Chloro-2-methylbenzene |
 |
m-Chlorotoluene | 1-Chloro-3-methylbenzene |
| p-Chlorotoluene | 1-Chloro-4-methylbenzene | |
 |
o-Dibromobenzene | 1,2-Dibromobenzene |
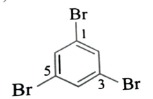 |
Tribromobenzene | 1,3,5-Tribromobenzene |
Nature of C—X Bond
- Due to electronegativity difference between carbon and halogen , shared pair of electron lie closer to halogen .
- Thus , Carbon bears a small positive charge and halogen bears a small negative charge . Hence , C—X bond is a polar covalent bond .
- As size of halogen atom increases , carbon halogen bond length increases and bond enthalpy decreases from C–F to C–I .
Preparation of Haloalkane
1) From Alcohols
The most convenient method for preparation of haloalkanes involves the substitution of the —OH group of an alcohol by the halogen atom .
a)By the Action of Halogen Acids
For a given alcohol , the reactivity of halogen acids decreases in order : HI > HBr > HCl and that of alcohols for a given halogen acid follows the order : tertiary > secondary > primary
R—OH (Alcohol) + HX → R—X (Haloalkane) + H2O
CH3CH2OH (Ethanol) + HCl CH3CH2Cl (Chloroethane) + H2O


The above reaction is not applicable for preparation of aryl halide because carbon- oxygen bond in phenol has a partial double bond character and is difficult to break being stronger than a single bond.
b)By the action of Phosphorous Halide
Phosphorous halide react with alcohol to form haloalkane or alkyl halide in excellent yields (80% or above).
ROH (Alcohol) + PCl5 (Phosphorous pentachloride) → R—Cl (Chloroalkane) + POCl3 (Phosphorous oxychloride) + HCl
CH3CH2OH (Ethanol) + PCl5 (Phosphorous pentachloride) → CH3CH2-Cl (Chloroalkane) + POCl3 + HCl
3ROH(Alcohol) + PCl3 (Phsphorous trichloride) → 3R-Cl (Alkyl chloride ) + H3PO3 (phosphoric acid)
3 

Similarily , bromoalkane and iodoalkane are prepared by the action of phosphorous tribromide and phosphorous tri-iodide respectively since PBr3 and PI3 are not very stable compound , these are generally prepared insitu by the action of red phosphorous on bromine and iodine respectively .
P4 +6Br2 →4PBr3 3CH3CH2CH2OH (Propan-1-ol) + PBr3 → 3CH3CH2CH2Br (1-Bromopropane) + H3PO3
P4 +6I2 →4PBr3 | 3CH3OH (Methanol) + PI3 → 3CH3I (Iodomethane) + H3PO3
c)By the Action of Thionyl Chloride
Chloroalkanes are conveniently prepared by refluxing alcohols with thionyl chloride in presence of pyridine . Thionyl chloride method is preferred to hydrogen chloride or phosphorous pentachloride for the preparation of chloroalkanes since both the by product SO2 and HCl in this reaction being gases escape leaving behind the chloroalkane in almost pure state.
ROH + SOCl 2 → R—Cl + SO2 ↑ + HCl ↑
CH3CH2OH (Ethanol) + SOCl2 → CH3CH2-Cl (Chloroalkane) + SO2 ↑ + HCl ↑
2) From Hydrocarbon
a)From Alkanes
Free radical Halogenation
Halogenation of alkanes with Cl2 or Br2 in presence of heat gives a complex mixture of mono , di and polyhaloalkanes.
CH4 (Methane) CH3Cl (Chloromethane) + CH2Cl2 (Dichloromethane) + CHCl3 (Trichloromethane) + CCl4 (Tetrachloromethane )
This reaction can be controlled if Cl2 is taken in limited amount .
In case of higher alkanes , even monohalogenation gives a mixture of all possible isomeric haloalkanes.
CH3-CH2-CH3 (Propane)CH3-CH2-CH2 –Cl[1-Chloropropane(45%)] +CH3-CHCl-CH3[2-Chloropropane(55%)]
Note :-Stability Order
- Free Radical : 3º Cº > 2º Cº > 1º Cº > -CH3 º
- Carbocation : 3º C+ > 2º C+ > 1º C+ > -CH3 +
- Carboanion : -CH3 – > 1º C – > 2º C– > 3º C–
Iodination
It is reversible but may be carried out in presence of an oxidising agent which destroys the HI as it is formed and thus drives the reaction in forward direction .
CH4 + I2 ↔ CH3I + HI ; 5HI + HIO3 → 3I2 + 3H2O
Fluorination
Alkyl fluorides are more conveniently prepared indirectly by heating suitable chloro- or bromoalkanes with inorganic fluorides such as AgF , Hg2F2 etc . This is called Swarts Reaction .
CH3Br + AgF → CH3F+ AgBr
2CH3-CH2 –Cl + Hg2F2 → 2CH3-CH2 –F + Hg2Cl2
b)From Alkenes
Addition of Halogen Acids (HX)
To Symmetrical Alkene : CH2 = CH2 + HI → CH3– CH2–I
The addition of hydrogen halides to unsymmetrical alkenes and alkynes takes place according to Markovnikov’s rule .
CH3—CH= CH2 + HBr
Addition of Halogen acid in presence of peroxide
However , in presence of peroxides such as benzoyl peroxide , the addition of HBr to unsymmetrical akene takes place by antimarkovnikov rule . This is known as peroxide effect or kharasch effect .
CH3—CH= CH2 + HBr CH3—CH2 –CH2Br
Allylic Halogenation
Reaction in which halogenation occurs at the allylic position of an alkene are called allylic halogenation reaction .
When alkene except ethylene are heated with Cl2 or Br2 at a high temperature of about 773K , then hydrogen atom at allylic carbon is replaced by halogen atom forming allyl halides .
CH3-CH=CH2 (Propene) + Cl2 → Cl-CH2-CH=CH2 (3-Chloroprop-1-ene) + HCl
CH3-CH=CH2 (Propene) + Br2 → Br-CH2-CH=CH2 (3-Chloroprop-1-ene) + HBr
Addition of Br2 in CCl4
vic-Dibromides can be prepared by addition of Br2 in CCl4 to alkenes
CH2 = CH2 + Br2 BrCH2—CH2Br (1,2-Dibromoethane)
When Br2 / CCl4 is added to alkene , the reddish brown colour of bromine is discharged due to formation of colourless vic-dibromide. Therefore , this reaction is used as test for detection of unsaturation in an organic molecule.
3) From Silver Salt of Fatty Acids
The decomposition of the silver salt of a carboxylic acid with Br2 in refluxing CCl4 to form an alkyl or aryl bromide with one carbon less than the original acid is called Hunsdiecker Reaction .
RCOOAg + Br2 R—Br + CO2 + AgBr
CH3-CH2 –COOAg + Br2 CH3-CH2 –Br (Ethyl bromide ) + CO2 + AgBr
C6H5COOAg + Br2 C6H5–Br (Bromobenzene) + CO2 + AgBr
4) By Halide Exchange
Finkelstein Reaction
Iodoalkanes can be easily prepared from the corresponding chloro- or bromoalkanes by heating with sodium iodide in acetone or methanol .
CH3-CH2 –Br + NaI CH3-CH2 –I (Iodoethane) + NaBr
This reaction is called Finkelstein Reaction . This reaction is very useful for preparing such alkyl iodides which cannot be prepared by direct addition of HI to alkenes.
Swarts Reaction
Alkyl fluorides are more conveniently prepared indirectly by heating suitable chloro- or bromoalkanes with inorganic fluorides such as AgF , Hg2F2 etc . This is called Swarts Reaction .
CH3Br + AgF → CH3F+ AgBr
2CH3-CH2 –Cl + Hg2F2 → 2CH3-CH2 –F + Hg2Cl2
Physical Properties of Haloalkanes
Physical State , Colour , Odour
Alkyl Halides are colourless when pure , but iodides develop colour when exposed to light. Methyl chloride , methyl bromide , ethyl chloride and some chlorofluoromethanes are gases at room temperature. Higher chloro , bromo , iodo compounds are either liquids or solids. Many volatile halogen compounds have sweet smell.
Melting Points and Boiling Points
- Due to greater polarity as well as higher molecular mass as compared to parent hydrocarbon , intermolecular force are stronger in halogen derivatives. Thus , boiling point of chlorides , bromides and iodides are comparitively higher than those of hydrocarbon of comparable molecular mass.
- For the same alkyl group , boiling point of haloalkane decreases in order : RI > RBr > RCl > RF . This is because with increase in size and mass of halogen , the magnitude of vander waal force of attraction decreases.
- For the same halogen atom , the boiling point increases with increase in size of alkyl group .
- For isomeric alkyl halides , the boiling point decreases with branching because with branching the surface area of the alkyl halide decreases and hence magnitude of vanderwaal force of attraction decreases.
- The boiling point of chloro , bromo and iodo compounds increase as the number of halogen atom increases.
Density
- Alkyl fluorides and chlorides are generally lighter than water whereas alkyl bromides and iodides and polyhalides are heavier.
- Among the alkyl halide , methyl iodide has highest density.
- As size of alkyl group increase , density of alkyl halides decreases.
Solubility
- Haloalkane are very slightly soluble in water. If a haloalkane is to dissolve in water , energy is required to overcome the forces of attraction already existing between haloalkane molecules and to break the hydrogen bond already existing between water molecules.
- However , these are quite soluble in organic solvents of low polarity because the new force of attraction set up between haloalkane and solvent molecules are of same strength as force of attraction being broken
Stability
Since the strength of C-X bond decreases in order : C–F > C–Cl > C–Br > C–I
Thus , stability of haloalkanes having same alkyl group decreases in order :
alkyl fluorides > alkyl chlorides > alkyl bromides > alkyl iodides
Chemical Reactions of Haloalkanes
Nucleophilic Substitution Reaction
Reaction in which a stronger nucleophile displaces a weaker nucleophile are called nucleophilic substitution reaction and the atom or group (halide ion in present case ) which departs with its bonding pair of electrons is called the leaving group . Better the leaving group , more facile is the nucleophilic substitution reaction .
Amongst the halide ions , the other in which the leaving groups departs follows the sequence :
I– > Br– > Cl– > F –
Substitution of Halogen by Hydroxyl Group (Formation of Alcohols)
Haloalkanes on treatment with boiling aqueous alkalies or silver oxide in boiling water undergo hydrolysis to form alcohols .
RX (Haloalkane) + KOH (aq) → R—OH (Alcohol) + KX
CH3CH2-Br (Bromoethane) + KOH (aq) → CH3CH2-OH (Ethanol) + KBr
CH3I (Iodomethane) + AgOH (aq) → CH3OH (Methanol) + AgI
Substitution of Halogen by Alkoxy Group
Haloalkanes when treated with sodium or potassium alkoxides form ethers or alkoxyalkanes.
R—X + NaOR’ → R—O—R’ + NaX
CH3CH2-Br (Bromoethane) +NaOCH2CH3 → CH3CH2 —O—CH2CH3 (Ethoxyethane) + NaBr
CH3I + NaOCH3 → CH3 —O—CH3 + NaI
This reaction is called Williamson synthesis.
Substitution of Halogen by Cyano Group
Haloalkanes or alkyl halides react with alcoholic solution of potassium cyanide to form alkyl cyanides or alkyl nitriles as the major product along with small amount of alkyl isocyanides.
R—X (Haloalkane) + KCN (alc.) → R—C≡N (Alkyl cyanide) + KX
CH3I + KCN → CH3C≡N (Methyl cyanide ) + KI
CH3CH2-Br + KCN → CH3CH2-C≡N (Ethyl cyanide) + KBr
Alkyl cyanides are very useful compounds since they can be converted into :-
i)amides on partial hydrolysis with conc. HCl or H2SO4 followed by treatment with H2O .
CH3 C≡N (Ethanenitrile) + H2O CH3 —CO—NH2 (Ethanamide)
ii)carboxylic acids on complete hydrolysis with dilute mineral acids or alkalies
CH3 C≡N (Ethanenitrile) + H2O CH3 COOH (Ethanoic acid) + NH3
iii)primary amines by reduction with sodium and alcohol (Mendius reduction ) or catalytically with H2/Ni or with lithium aluminium hydride
CH3 C≡N (Ethanenitrile) + 4 [H] CH3 CH2—NH2 (Ethanamine)
Substitution of Halogen by Isocyanide Group
When the aqueous ethanolic solution of a haloalkane is heated with silver cyanide, alkyl isocyanide or alkyl isonitrile or alkyl carbylamine is produced as the chief product along with a small amount of the corresponding alkyl cyanide.
R—X + Ag—CN R—NC+AgX
CH3I + Ag—CN CH3NC + AgI
Substitution of Halogen by Nitrile Group
Haloalkanes react with sodium or potassium nitrile to form alkyl nitriles.
R—X + KNO2 → R—O—N≡O (Alkyl nitrile) + KX
CH3CH2-Br + KNO2 → CH3CH2–O—N≡O (Ethyl nitrile) + KBr
Substitution of Halogen by Nitro Group
When an aqueous ethanolic solution of a haloalkane is heated with silver nitrile , a nitroalkane is formed.
R—X + Ag—O—N: = O

CH3CH2-Br + KNO2 → CH3CH2-NO2 (Nitroethane)+ AgBr
Note : Here KONO is mainly ionic so attack takes place by oxygen atom which is electron rich whereas AgNO2 is mainly covalent so attack takes place by Nitrogen atom which has lone pair.
Substitution of Halogen by Alkynl Group
When treated with sodium alkynides , haloalkanes form higher alkynes .
CH3CH2-Br (Bromoethane) + CH≡CNa (Sod. acetylide) → CH3CH2—C≡CH (But-1-yne) + NaBr
Mechanism of Nucleophilic Substitution Reaction
a)SN2 (Substitution , nucleophilic , bimolecular)
- The reaction follows second order kinetics, i.e., the rate of reaction depends upon the concentration of both the reactant.
- When incoming nucleophile approaches the alkyl halide molecule and starts interacting with it , the carbon- halogen bond starts breaking a new carbon- nucleophile bond starts forming.
- These two process occur simultaneously and no intermediate is formed .
- Such two reaction occur through a transition state in which both the reactant are partially bonded to each other.
- In SN2 reaction , the attack of nucleophile occurs from the back side and the leaving group leaves from front side.
- Thus SN2 reaction are always accompained by inversion of configuration .
- Rate of reaction is inversely proportional to steric hinderance
- The order of reactivity follows the sequence : Methyl > 1º > 2º > 3º alkyl halides
- Polar aprotic solvent of high dielectric constant favours SN2 reaction.
b)SN1 (Substitution , nucleophilic , unimolecular)
- The reaction between tert-butyl bromide and OH– ion to yield tert-butyl alcohol and Br– ion follows first order kinetics, i.e., the rate of reaction depends upon concentration of tert-butyl bromide only and is independent of concentration of OH- ions.
- The reaction occurs in two steps .
- In first step ; tert-butyl bromide undergoes ionization to produce tert-butyl carbocation and a bromide ion . The step is slow and hence is rate determining step of reaction .
- In the second step , carbocation being a reactive chemical species is immediately attacked by nucleophile . This step is fast and does not affect the rate.
- Carbocations are intermediates.
- Both backside and frontside attack of the nucleophile occurs through which the backside attack predominates.
- Low concentration of nucleophiles generally favours them .
- Polar protic solvents of high dielectric constants favours SN1 reaction .
- The order of reactivity follows the sequence : 3º > 2º >1º > methyl halide.
- Electronic factors affect the reaction rates greatly.
Reaction with Metals
Reaction with Mg
R—X (Alkyl halide ) + Mg R—MgX (Grignard reagent )
CH3CH2-Br + Mg CH3CH2-MgBr (Ethylmagnesium bromide)
In Grignard reagent , carbon – halogen bond is covalent but highly polar due to large electronegativity difference between carbon and magnesium . The magnesium – halogen bond is ionic .
Due to high polarity , Grignard reagent are a potential source of carbanions and hence are very reactive. Thus, It is necessary to protect grignard reagent from moisture otherwise these will react with water to form hydrocarbons.
Wurtz Reaction
It involves the interaction of two molecules of an alkyl halide (preferably bromide or iodide ) with metallic sodium in presence of dry ether to form symmetrical alkanes containing double the number of carbon atom present in the alkyl halide.
R—X (Alkyl halide ) + 2Na + X—R R—R (Alkane) + 2NaX
CH3—Br + 2Na + Br—CH3 CH3—CH3 (Ethane) + 2NaBr
CH3CH2—I + 2Na + I—CH2CH3 CH3CH2—CH2CH3 (n-Butane)
Wurtz reaction is a convenient method for preparation of symmetrical alkanes , i.e., alkanes containing even number of carbon atoms.
Elimination Reaction : Dehydrohalogenation
When a haloalkane is heated with a concentrated alcoholic solution of potassium hydroxide , a molecule of hydrogen halide is eliminated and an alkene is formed.
The hydrogen of alkyl halide which is eliminated comes from a β – carbon and the halogen from the α-carbon.
CH3CH2CH2CH2Cl (1-Chlorobutane) + KOH (alc.) → CH3CH2CH=CH2 (But-1-ene) + KCl + H2O
If the halogen is present on any carbon atom within the chain , the alkyl halide can undergo dehydrohalogenation in two or more different directions . In all such cases , the more highly substituted alkene is the major product of dehydrohalogenation . This generalisation is known as Saytzeff’s rule and this mode of elimination is called Saytzeff elimination.
Haloarenes
Preparation of Haloarenes
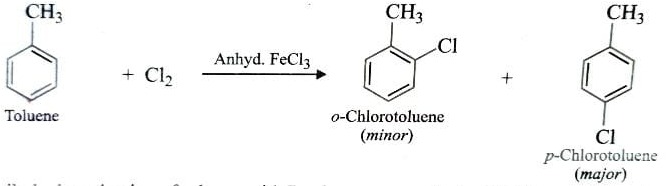
By Direct Halogenation
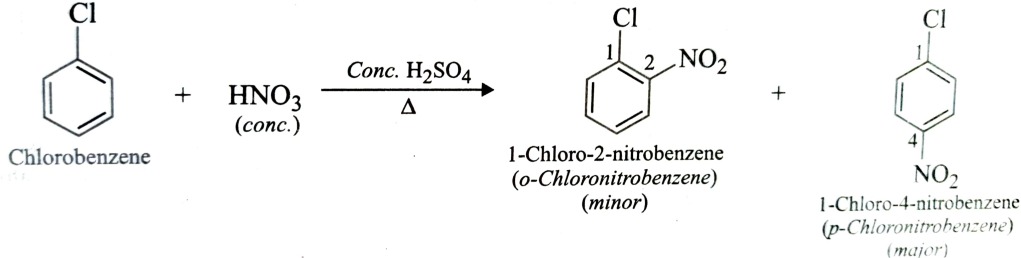
Chloroarenes and Bromoarenes can be prepared by direct chlorination or bromination of aromatic hydrocarbons.
This reaction is usually carried out in absence of sunlight and in presence of lewis acid such as anhydrous ferrous or aluminium halide as catalyst.
If toluene is used instead of benzene , a mixture of o-chlorotoluene (minor) and p-chlorotoluene(major) is obtained since CH3 group is o,p – directing .
By Side Chain Halogenation
When Cl2 is passed through boiling toluene in presence of sunlight and absence of halogen carrier , phenylchloromethane is formed.
From Diazonium Salt by Sandmeyer Reaction
Formation of Diazonium Salt is as follows:
The conversion of benzenediazonium chloride to chlorobenzene , bromobenzene and benzonitrile on treatment with CuCl/HCl , CuBr/HBr or CuCN/KCN respectively is called Sandmeyer reaction
However , iodoarenes are prepared by simply warming the diazonium salt solution with aqueous KI solution.
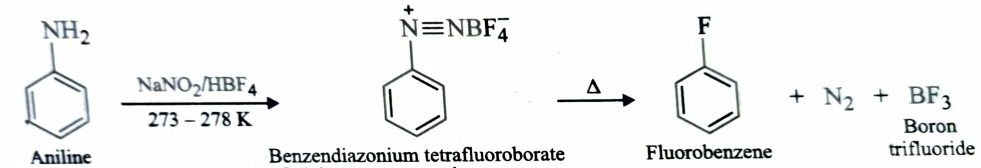
Balz- Schiemann Reaction
Fluoroarenes are prepared by heating the corresponding diazonium tetrafluoroborates which in turn are obtained by diazotisation of suitable aromatic primary amine with aqueous NaNO2 in presence of fluoroboric acid (HBF4) at 273-278K . This reaction is called Balz – Schiemann reaction.
By Gattermann Reaction
This is a modification of Sandmeyer reaction in which benzenediazonium chloride is treated with copper powder and a halogen acid (instead of cuprous halide dissolved in the corresponding halogen acid) to form aryl halides.
Physical Properties of Haloarenes
Melting Points and Boiling Points
- The boiling point of haloarenes are nearly same as those of haloalkanes with the same number of carbon atoms.
- For the same aryl group , the melting point and boiling point increases as size of halogen atom increases.
- For the same halogen atom , the melting and boiling point increases as the size of aryl group increaes.
- The boiling point of isomeric dihalobenzene are nearly same but their melting point are quite different. The melting point of p- isomer is always higher than o- and m- isomer since p-isomer is more symmetrical and hence its molecules pack close in the crystal lattice.
Density
All aryl halides are heavier than water.
Chemical Reaction of Haloarenes
Nucleophilic Substitution Reaction
Why Haloarenes are extremely less reactive than haloalkanes toward nucleophilic substitution reaction ?
- Resonance Effect : In haloarenes lone pair of electron on halogen atom are delocalized on the benzene ring . As a result , C—Cl bond acquires partial double bond character whereas in case of alkyl halides carbon is attached to chlorine by a pure single bond. Thus , C—X bond in aryl halides is stronger than alkyl halides and cannot be easily broken.
- Difference in hybridization of carbon atom in C—X : In haloarenes halogen is attached to sp2 hybridized carbon while in haloalkanes , halogen is attached to sp3 hybridized carbon. C—Cl bond in haloarene should be shorter (due to small size of sp2 hybridized orbital) and hence stronger than haloalkane. Thus , C—Cl bond in haloarene is stronger than haloalkane.
- Instability of phenyl cation : In haloarene , phenyl cation formed as a result of self ionization is not stabilized by resonance. Hence aryl halides does not easily undergo nucleophilic substitution reaction .
Replacement by Hydroxyl Group
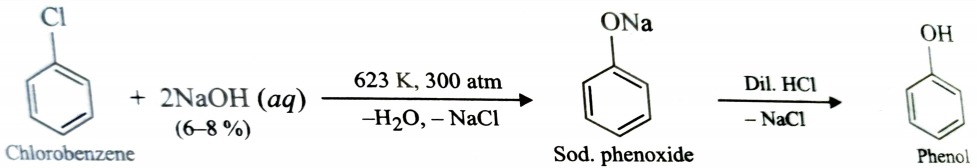
Due to low reactivity , nucleophilic substitution reaction in aryl halides occur under drastic condition. Chlorobenzene when treated with aqueous solution of NaOH at 623 K under 300atm pressure, gives sodium phenoxide which upon acidification gives phenol . This reaction is called Dow’s process .
Further , presence of electron withdrawing group at o and p position activates the halogen towards nucleophilic displacement. Moreover , greater the no of such groups more reactive is the haloarene.
Electrophilic Substitution Reaction
Haloarenes undergo the usual electrophilic substitution reaction of the benzene ring such as halogenation , nitration , sulphonation and electrophilic substitution reaction . Halogen atom is slightly deactivating and o,p-directing .
Halogenation
Nitration
Sulphonation
Friedel Craft Reaction
Reaction with Metal
Wurtz Fittig Reaction
Haloarenes when treated with an ethereal solution of alkyl halide in presence of sodium , form alkyl derivative of benzene. This is called wurtz fitting reaction.
Fittig Reaction
When only haloarenes are treated with sodium , diaryls are produced. This reaction is called Fittig reaction .
Reaction with Magnesium
Bromo and iodoarenes form grignard reagent when their ethereal solution is treated with magnesium turnings.
Polyhalogen Compounds
Compounds containing more than one halogen atom are called polyhalogeno compound.
Dichloromethane (Methylene chloride )
- It is widely used as solvent for paint remover , as a propellant in aerosols and as a process solvent in manufacture of drugs.
- Exposure to some level of methylene chloride in air causes slightly impaired hearing and vision.
- High level of methylene chloride in air causes dizziness , nausea , tingling and numbness in fingers and toes.
Trichloromethane (Chloroform)
Preparation : CCl4 + 2[H] CHCl3 (Chloroform ) + HCl
Physical Properties : It is heavy colourless mobile liquid . It has sticky sweet smell nd burning taste. It is sparingly soluble in water but much readily dissolves in alcohol and ether. It is very good solvent for organic compound.
Chemical Properties :
- When Chloroform is exposed to sunlight and air , it decomposes into phosgene .
2CHCl3 + [O] → 2COCl2 (phosgene) + 2HCl
- Chloroform may be nitrated on heating with concentrated nitric acid to give chloropicrin (tear gas) .
CHCl3 + HONO2 → CCl3NO2 (Chloropicrin)(Tear gas) + H2O
Triiodomethane
It is a pale yellow solid having a characteristic odour
Preparation : CH3CH2OH + 4I2 + 6NaOH → CHI3 + HCOONa + 5NaI + 5H2O
Its antiseptic property is due o liberation of iodine when iodoform comes in contact with skin but not due to iodoform itself.
Tetrachloromethane (Carbon tetrachloride )
- It is produced in large quantities by of methane in presence of light.
- It is used in manufacture of refrigerant.
- It is also used as solvent in manufacture of pharmaceuticals.
- Exposure of carbon tetrachloride causes liver cancer in humans.
- When carbon terachloride is released into air , it goes into upper atmosphere and depletes the ozone layer.
Freons
Chlorofluoro compounds of methane and ethane in which all the H-atoms are replaced by halogen atom are collectively called freons.
Preparation : 3CCl4 + 2SbF3 → 3CCl2F2 (Freon-12) + 3SbCl3
It is an odourless , non-corrosive , non toxic gas which is stable even at high temperature and pressure.
It is widely used as cooling agent in refrigerators and air conditioners.
# Haloalkanes and Haloarenes Class 12 Notes
# Haloalkanes and Haloarenes Class 12 Notes in Detail
# Haloalkanes and Haloarenes Class 12 Notes With NCERT Solutions
# Haloalkanes and Haloarenes Class 12 Notes with Questions
# Haloalkanes and Haloarenes Class 12 Notes of the chapter
Do share the post if you liked Haloalkanes and Haloarenes Class 12 Notes. For more updates, keep logging on BrainyLads


















Nice ❤️