Electrochemistry Class 12 | Chapter 3 | Chemistry | CBSE |
Table of Contents
Electrochemistry Class 12 | Chapter 3 | Chemistry | CBSE |
Electrochemistry
Electrochemistry Class 12 : Electrochemistry deals with the relation between electrical energy and chemical energy and with method of their inter-conversion. It is the study of production of electricity from energy released during spontaneous chemical reaction and the use of that electricity to bring out non- spontaneous chemical transformation.
Conductors : Substance which allow electric current to flow through it .
Types of Conductors
|
Metallic or Electronic Conductors |
Electrolytic Conductors |
| Conduct electric current due to mobility of free electrons in a particular direction when a potential difference is applied across ends of conductor. | Conduct electric current due to mobility of free ions ( cation and anion ). These substances are chemically decomposed as electrolytes. |
| Conductance decreases with increase in temperature due to vibration in kernels which decrease mobility of electrons. | Conductance depends on mobility of free ions and extent of ionization. |
Types of Electrolytes
1)Strong Electrolytes : The electrolytes which are completely ionized into ions in molten and in solution are called as strong electrolytes. Ex : KCl , HCl
Its conductance depend only upon mobility of free ions.
2) Weak Electrolytes: The electrolytes which are partially ionized into ions in solution are called weak electrolytes. Ex : NH4OH , ZnCl2 .
Its conductance depends upon mobility of free ions and degree of ionization.
Note: Increase in conductance of weak electrolyte is more than increase in conductance of strong electrolyte.
Electrolysis
A process of decomposition by passage of electricity through its aqueous solution or molten state is called Electrolysis.
Primary Change
The conversion of ions into neutral species or gaining or losing of electrons takes place at their respective electrodes is called primary change.
Secondary Change
The product formed due to primary change is either may be collected or may undergo a secondary change to form final product.
Electrolysis of Molten NaCl
NaCl → Na+ + Cl–
At anode : Cl– → Cl• + e– (Primary change)
Cl• + Cl• → Cl 2↑ (Secondary change)
At Cathode : Na+ + e– → Na (Primary change)
Overall Reaction : 2Na+ + 2 Cl– → 2 Na + Cl 2
So, Na metal is deposited at cathode and Cl2 gas is liberated at anode.
Electrolysis of Molten NaCl
Here NaCl and H2O both are ionized
NaCl → Na+ + Cl–
H2O ↔ H + + OH – {Here preferential order theory is applied. }
At Anode : Cl– – e– → Cl• (Primary change)
Cl• + Cl• → Cl 2↑ (Secondary change)
Here, Cl – has low discharge potential than OH–.
At Cathode : H+ + e– → H• (Primary change)
H • + H• → H2↑ (Secondary change)
Here , H+ has lower discharge potential than Na+
Faraday’s Law of Electrolysis
Faraday First Law of Electrolysis
It states that the amount (mass/volume) of a substance which is deposited or liberated at any electrode is directly proportional to the supplied charge or quantity of electricity passes through electrolyte.
W ∝ Q
W = Z .Q { Here Z is electrochemical equivalent constant }
W = Z I T { Q = I t }
Electrochemical equivalent of a substance is the amount of substance liberated or deposited when one coulomb of electricity is passed through electrolyte. {W = Z}
Unit of Z = W / Q i.e. g/coulomb.
Faraday Second Law of Electrolysis
It states that when same amount of electricity is passed through different electrolyte connected in series , the amount of liberated or deposited substance of electrode is directly proportional to their equivalent mass.
Amount of Ag deposited (w1) / Amount of Cu deposited (w2) = Equivalent Mass of Ag (E1) / Equivalent mass of Cu (E2)
i.e ; w1 / w2 = E1 / E2
Z1 I T / Z2 I T = E1 / E2
Z1 / Z2 = E1 / E2
Thus , E ∝ Z or E = F Z { F = 96500}
Here, F = Faraday Constant
1F = 96500C of electricity deposits one gram equivalent of the electrolyte.
Note : 1 F = charge on an electron × Avogadro no
1 F = 1.6 x 10-19 x 6.022 x 1023 = 96500 Cmol-1
W = Z x Q = (E / F ) x Q = I x T x E / Q { E = Equivalent Mass = atomic mass / valency = M / Z }
W = I x T x M / F x Z
Conductance of Electrolytic Solution
Ohm’s Law: It states that current I that flows through a conductor is directly proportional to the potential difference.
I ∝ V
I = 1/R × V
V = I R
Resistance
It is the property of substance which obstructs the flow of current through it .
Factors Whereon Resistance depends
R ∝ L ( Length)
R ∝ 1/ A ( Area of Cross Section )
R = ρ L / A { Here; ρ = resistivity}
Specific Resistance or Resistivity
It is defined as resistance offered by a conductor of 1 cm length with 1 cm2 area of cross- section. Ρ = RA / L
Unit of resistivity is ohm cm .
Conductance
It is denoted by G. It is reciprocal of resistance. G = 1 / R
Unit of conductance is Siemens or mho or ohm-1.
Specific Conductance or Conductivity
It is reciprocal of resistivity . It is denoted by Ƙ
κ = 1 / ρ
κ = (1 / R) x L /A
κ = G x L /A
Conductivity is the conductance of 1cm3 of the electrolytic solution.
Its unit is S cm-1.
Cell Constant
Conductivity depends upon conductance and l and a . But the measurement of l and a is neither convenient nor reliable. So for a particular cell L / A is constant and this constant is cell constant .
G ∗ = L / A κ = G x G ∗
Equivalent Conductivity
Equivalent conductivity of a solution at a dilution V is defined as conductance of all ions produced from 1 g equivalent of an electrolyte dissolved in V cm3 of solution when distance b/w electrode is 1cm and area of cross section of electrode is 1cm2. It is denoted by Ʌeq .
Its Unit is ohm-1 cm2 g-1 eq-1
Λeq = κ × 1000 / Normality (N)
Molar Conductivity
It is the conductance of all ions produced by one gram mole of electrolyte in solution
Λm= κ × 1000 / Molarity (M)
Its unit is S cm2 mol-1 .
Variation of Conductance, Molar Conductivity, Equivalent Conductivity and Specific Conductance with Concentration or Dilution
- Conductance of a solution is due to presence of ions in solution . The greater the number of ions, the greater is the conductance. As on dilution , more ions are produced in solution, so the conductance should also increase on dilution.
- Conductivity or specific conductance always decreases with decrease in concentration for both weak as well as strong electrolyte.
- Molar conductivity of Strong Electrolyte : Ʌmc vary with concentration according to Debye Huckel Onsager equation : Ʌmc = Ʌmo ─ A√C . For a strong electrolyte, there is only a small increase in conductance with dilution. This is because a strong electrolyte is completely dissociated in solution and so number of ions remains constant.
- Molar conductivity of weak electrolyte : There is a very large increase in conductance with dilution especially near infinite dilution because on decreasing concentration , weak electrolyte starts to dissociate into ions and also mobility increases due to increase in volume.
- Equivalent conductivity also increases upon dilution.
Kohlraush’s Law
The limiting molar conductivity of an electrolyte is the sum of the limiting ionic conductivities of the cation and the anion each multiplied with the number of ions present in one formula unit of the electrolyte.
1) Calculation of Λºm or Λ m∞ for weak electrolyte
Consider the example of acetic acid CH3COOH as weak electrolyte .
By Kohlraush ‘s Law : Λºm (CH3COOH) = λºm (CH3COO–) + λºm (H +)
The above equation can be calculated by achieved by knowing Λºm values of strong electrolyte KCl , CH3COOK and HCl .
Λºm (KCl ) = λºm (K +) + λºm (Cl – ) ….. (i)
Λºm ( CH3COOK ) = λºm (CH3COO– ) + λºm (K +) …..(ii)
Λºm (HCl ) = λºm (H +) + λºm (Cl – ) ….. (iii)
Adding (ii) and (iii) and subtracting (i) ;
λºm (CH3COO– )+λºm (K +) + λºm (H +) + λºm (Cl – ) – λºm (K +) – λºm (Cl – ) = λºm (CH3COO–) + λºm (H +)
Λºm ( CH3COOK ) + Λºm (HCl ) – Λºm (KCl ) = Λºm (CH3COOH)
2) Calculation of degree of dissociation of weak electrolyte
Degree of dissociation , α = Λcm / Λºm
Here , Λcm is molar conductivity at given concentration .
Λºm is molar conductivity of infinite dilution.
3) Calculation of dissociation constant of weak electrolyte
Consider CH3COOH as weak electrolyte , then
CH3COOH ↔ CH3COO– + H +
Initial Concentration : c 0 0
Equilibrium concentration : c – cα cα cα
Total concentration : c – cα + cα + cα = c (1 +α)

Num : Calculate the degree of dissociation of acetic acid if its molar conductivity is 39.05 Scm2mol-1 and λºm (CH3COO–) = 40.9 Scm2mol-1 ?
Soln : α = Λcm / Λºm
Λºm (CH3COOH) = λºm (CH3COO–) + λºm (H +) = 40.9 + 349.6 = 390.5 Scm2mol-1
Λcm = 39.05
∴ α = 0.1
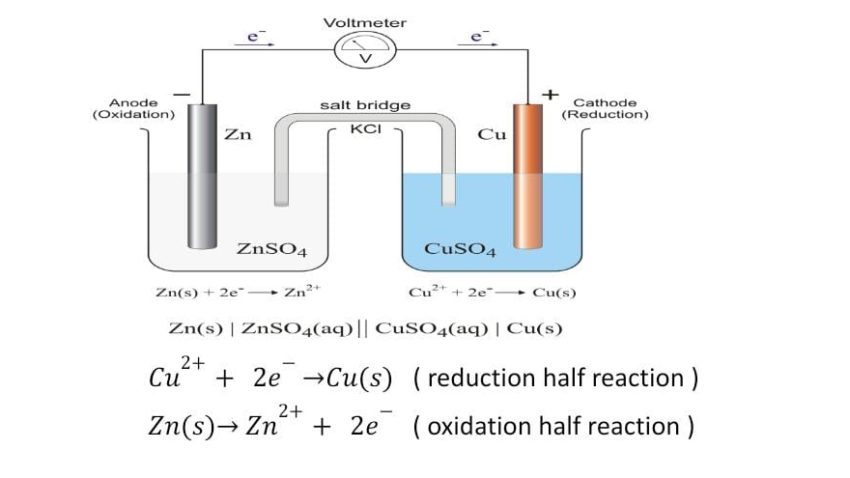
Electrochemical Cell
It is a device which is used to convert chemical energy of a spontaneous Redox reaction into the electrical energy. Such a device is also called Galvanic or Voltaic cell.
- The reduction half reaction occurs on copper electrode and oxidation half reaction occurs on zinc electrode.
- Since electrons are produced at the zinc electrode , this electrode is rich in electrons and pushes the electrons into the external circuit and hence it is designed as negative pole.
- The other electrode i.e. copper electrode is in need of electrons for reduction of Cu2+ into Cu i.e. this electrode is deficient in electrons and pulls the electrons from the external circuit, therefore it acts as positive pole.
- Deflection in voltmeter towards left indicates that current flows towards left
- The two electrolytes in respective beakers also called half cells remains electrically neutral.
Salt Bridge
It is an inverted U tube which contain suitable salt. It completes the electrical circuit by allowing the ions to flow from one solution to other without mixing of two solution . It also maintain electrical neutrality of solution in two half cells.
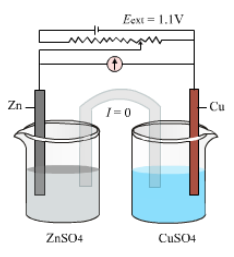
If an external potential is applied in galvanic cell and increased slowly then reaction continues to take place until opposing voltages reaches 1.1V.
- When Eext = 1.1V no current flows and no chemical reaction takes place.
- When Eext > 1.1 V electrons flow from Cu to Zn and current flows from Zn to Cu. Zinc is deposited at the zinc electrode and copper dissolves at copper electrode.
- When Eext < 1.1V , electrons flow from Zn rod to Cu rod . Hence current flows from Cu to Zn . Zn dissolves at anode and Cu dissolves at cathode.
Electrode Potential
The potential difference which is present between metal electrode and its ions in solution is called electrode potential.
Standard Electrode Potential
The electrode potential measured under standard condition of temperature (298 K) and for 1M concentration of ions in solution is called standard electrode potential. It is denoted by Eo.
Measurement of Standard Electrode Potential
We cannot measure the absolute Eo of an electrode. To solve this, we use a reference electrode called standard hydrogen electrode (SHE).
Standard Hydrogen Electrode
It is an electrode of platinum foil dipped in 1M H+ at 298K in which H2 gas at 1 atm. Is passed . Its standard electrode potential is taken as 0.
Electrochemical Series
It is an arrangement of all electrodes in order of their increasing standard electrode potential. .
Reduction Potential = – Oxidation Potential
| Lithium | Li +(aq) + e– → Li (s) | -3.03 |
| Potassium | K +(aq) + e– → K (s) | -2.92 |
| Calcium | Ca 2+(aq) + 2 e– → Ca (s) | -2.87 |
| Sodium | Na +(aq) + e– → Na (s) | -2.71 |
| Magnesium | Mg 2+(aq) + 2 e– → Mg (s) | -2.37 |
| Aluminium | Al 3+(aq) + 3 e– → Al (s) | -1.66 |
| Zinc | Zn 2+(aq) + 2 e– → Zn (s) | -0.76 |
| Iron | Fe 2+(aq) + 2 e– → Fe (s) | -0.44 |
| Lead | Pb 2+(aq) + 2 e– → Pb (s) | -0.13 |
| Hydrogen | 2H +(aq) + 2 e– → H2 (s) | 0.00 |
| Copper | Cu 2+(aq) +2 e– → Cu (s) | +0.34 |
| Silver | Ag +(aq) + e– → Ag (s) | +0.80 |
| Gold | Au 3+(aq) + 3 e– → Au (s) | +1.50 |
Application of Electrochemical Series
1) Comparison of reducing power of metal: Greater the negative value of standard reduction potential , more is reducing power of metal.
2) Comparison of oxidizing power of non- metal : Greater the positive value of standard reduction potential , more is oxidizing power of metal.
3) Comparison of thermal stability of metallic oxides : Greater the value of standard reduction potential , lesser is the stability .
4) Displacement reaction : Metals having negative value of standard reduction potential easily evolves H2 gas from dilute acid upon reaction.
A non metal with greater standard reduction potential can displace other non metal from solution.
5) Comparison of Electropositive nature of metals:
- Metals having standard reduction potential value near -2 V or more –ve are highly electropositive or reactive metals. For e.g. : Alkali / Alkaline Earth metals
- Metals having standard reduction potential between 0 V to -2 V are moderately electropositive. For e.g. Co , Ni , Mn , Zn
- Metals like Cu , Hg , Pt having +ve value of electropositive are weakely electropositive or less reactive metals .
6) Calculation of standard EMF of cell:
Eocell ( std. emf of cell) = Eocathode (std. redn pot. Of cathode) ─ Eoanode (std. red pot of anode)
7) Predicting the feasibility of cell:
Eocell = +ve (feasible or spontaneous reaction)
Effect of Concentration and Temperature on Single Electrode Potential and on Emf of Cell :
Nernst Equation : It is used to calculate electrode potential for concentration and pressures other than standard state condition.
Nernst equation for single electrode :
Let Mn+(aq) + ne– → M(s)
Nernst Equation for Cell Potential
Let us consider a Daniel Cell : Zn (s) + Cu 2+ (aq) → Zn 2+ (aq) + Cu (s)
Acc to Nernst Equation ;

Let a general equation be aA + bB → xX + yY
Equilibrium Constant for Nernst Equation
Let us consider the following cell
Zn (S) |ZnSO4 || CuSO4 | Cu(s)
Zn (s) + Cu 2+ (aq) → Zn 2+ (aq) + Cu (s)
At equilibrium : Ecell = 0

Gibb’s Free Energy
Electrical work done = Electrical energy produced
W= quantity of charge flowing × Emf
Decrease in free energy = Wmax = n × F x ECELL
-G = Wmax = n × F x ECELL
ΔG = -n F ECELL { this formula is used if standard condition are not given }
For standard condition : ΔGo = -n F E o CELL
Relation Between ΔGo and K C
Numerical : The emf of the following cell is found to be 0.20V at 298K Cd|Cd2+ (?) || Ni2+ (2.0M) |Ni . What is the molar concentration of Cd2+ ions in the solution ?

Soln : The cell reaction is Cd + Ni2+ → Cd2+ + Ni
log10Cd2+ ─ log10 2 = ─1.690
log10Cd2+ = ─1.690 + 0.3021
[Cd2+ ] = antilog 2.6121 = 0.0409M
Num : At what pH of HCl solution will hydrogen gas electrode show electrode potential of -0.118 V ? H2 gas is bubbled at 298K and 1 atm pressure.
Soln : H+ + e– → 1/2 H2
─0.118 = ─0.0591 pH
PH = 2
Batteries
The combination of two or more cells connected in series. It is also called Commercial Cell.
Types of Batteries or Commercial Cell
1) Primary Batteries
Primary cell are those in which redox reaction occur only once i.e The cell in which product cannot be changed back into reactant . eg : Dry cell, mercury cell .
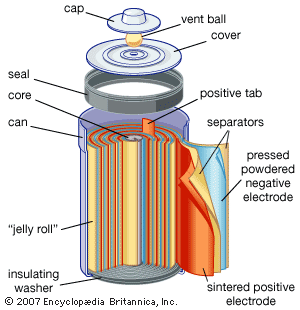
Dry Cell
It consists of Zinc container which acts as anode and cathode is a carbon (graphite ) rod surrounded by powdered manganese dioxide and carbon. The space b/w electrodes is filled by moist paste of NH4Cl and ZnCl2 .
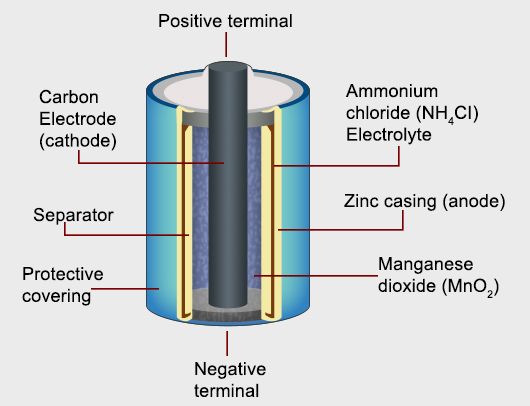
At Anode : Zn (s) → Zn 2+ + 2 e–
At Cathode : MnO 2 + NH 4 + + e– → MnO (OH) + NH 3
At cathode , manganese is reduced from +4 to +3 state . Ammonia produced in the reaction form complex with Zn2+ to give [Zn(NH3)4]2+ . The cell has potential of nearly 1.5V , it decreases with time .
Mercury Cell
It consists of Zn – Hg amalgam as anode . The cathode is a paste of mercuric oxide (HgO) and carbon powder . The electrolyte is a paste of KOH and ZnO.
At Anode : Zn (Hg) + 2OH– → ZnO (s) + H2O + 2e–
At Cathode : HgO + H2O + 2e– → Hg(l) + 2OH–
The cell potential is approximately 1.35 V and remains constant during its life .
2) Secondary Cells
A secondary cell is capable of being charged after discharging again and again. E.g. lead storage battery , nickel- cadmium battery
Lead Storage Battery
It consists of a lead anode and a grid of lead packed with lead dioxide as a cathode. A 38% solution of sulphuric acid acts as the electrolyte. When the battery is in use (discharging) , the cell reactions are
At Anode : Pb(s) + SO42- (aq) → PbSO4 + 2e–
At cathode : PbO2+ SO42- + 4H+ +2e– → PbSO4 + 2H2O
Overall reaction : Pb(s) + PbO2 + 2H2SO4 → 2PbSO4(s) + 2H2O
Nickel – Cadmium Cell
The overall cell reaction of Ni-Cd cell during discharging is-
Cd(s) + 2Ni(OH)3 (s) → CdO(s) + 2Ni(OH)2 + H2O It is another important secondary cell.
3) Fuel Cells
They are galvanic cells that are designed to convert the energy of combustion of fuels like hydrogen , methane , methanol directly into electrical energy . e.g. hydrogen – oxygen fuel cells , in which hydrogen and oxygen are bubbled through porous carbon electrodes into conc.
KOH solution
At Anode : 2H2 + 4 OH – → 4 H 2 O + 4 e–
At Cathode : O 2 + 2 H 2 O + 4 e– → 4 OH –
The overall reaction is 2H2 + O 2 → 2 H 2 O Corrosion
It is basically an electrochemical phenomenon in which a metal oxide or other salt of metal forms a coating on the metal surface . e.g rusting of iron . Rust is hydrated ferric oxide Fe2O3.x H2O
Oxidation at anode : 2Fe → 2Fe2+ + 4e–
Reduction at cathode : O2 + 4H+ + 4e– → 2 H2 O
Factors which increase Corrosion
- Presence of impurities
- Air and Moisture
- Presence of Electrolytes
- Uneven metal surface
Methods used for prevention of Corrosion
- By keeping the surface of the metal smooth
- By coating the metal surface with the film of oil and grease
- By painting the surface of metal
- By coating with more reactive metal i.e sacrificial protection
# Electrochemistry Class 12
# Electrochemistry Class 12 Notes
# Electrochemistry Class 12 Solutions
# Electrochemistry Class 12 Solutions NCERT
# Electrochemistry Class 12 Class 12 Solutions and Notes
# Notes of Electrochemistry Class 12
# Electrochemistry Class 12 Extra Questions
Do share the post if you liked Electrochemistry Class 12. For more updates, keep logging on BrainyLads