Solutions Chemistry Class 12 | Chapter 2 | Chemistry | CBSE | Term 1 |
Table of Contents
Solutions Chemistry Class 12 | Chapter 2 |
Solutions
Solutions Chemistry Class 12 : Solution is defined as homogenous mixture of two or more chemically non-reacting substance whose composition can be varied within in certain limits. Note: Solution have two components : solute and solvent
Solute (B) : The component of solution which is present in lesser amount by mass. The substance which is dissolved is also known as solute.
Solvent (A): The component of solute which is present in larger amount by mass . The substance in which the solute is dissolved is called solvent.
Example: Salt solution , Sugar solution , Syrup.
Types of Solution Based on Physical State of Solute and Solvent
| Type of solution | Solute | Solvent | Example |
| Solid solution | Solid | Solid | Alloys |
| Solid solution | Liquid | Solid | Amalgam of mercury with Na |
| Solid solution | Gas | Solid | Gases in minerals |
| Liquid Solution | Solid | Liquid | Salt in water |
| Liquid Solution | Liquid | Liquid | Ethanol in water |
| Liquid Solution | Gas | Liquid | Carbonated drinks |
| Gaseous solution | Solid | Gas | Camphor in N2 |
| Gaseous solution | Liquid | Gas | Humidity in air |
| Gaseous solution | Gas | Gas | Air |
Expressing Concentration of Solution
Concentration of solution is the amount of solute dissolved in known amount of solute or solvent.
- Mass Percentage (w/w): Amount of solute in gram present in 100g of solution .
Mass% of a component = (Mass of component in solution) / (Total mass of solution) x 100
- Volume Percentage (V/V) : It is defined as volume of solute in ml present in 100 ml of solution.
Volume % of a component = (volume of component in solution) / (total volume of solution) x 100
- Mass by volume percentage (w/V): It is defined as amount of solute in gram present in hundred ml of solution.
w/v % = (mass of solution) / (volume of solution) x 100
Note: 10% salt solution w/V means 10g of salt is present in 100ml of solution.
- Parts Per Million (ppm) : The number of parts by mass or volume of one component present in parts by mass or volume of solution.
Parts per million = Number of parts of component / total number of parts of all component in solution x 100 This term is used for calculation of pollutant concentration in air. It is also used to calculate hardness of water.
- Mole Fraction : Mole fraction of a component is the ratio of no of moles of that component to total moles of all components.
Mole fraction of solvent , xA = nA / nA + nB
lly ; Mole fraction of solute , xB = nB / nA + nB
Note : Sum of all mole fraction is unity.
- Molarity : It is defined as number of moles of solute present in per litre volume of solution. Its unit is molL-1 or Molar .
Molarity (M) = wB / MB x V (L)
- Molality : It is defined as no of moles of solute present in 1000g or 1kg of solution. It is temperature dependent. Its Unit is mol kg-1 OR molal. Molality (m) = no of moles of solute / mass of solute (kg)
It is temperature independent as mass does not depend on temperature .
- Normality : It is defined as number of gram equivalent of solute dissolved in per litre of the solution . It is temperature dependent . Its unit is Normal OR gm equivalent / litre.
Normality = No. of gram equivalent of solute / volume of solution in litre
Note: No. of gram equivalent of solute = Given mass / Equivalent mass
Equivalent mass of substance is defined as the number of parts by mass of it which combine with or displace directly or indirectly 1.008 by mass of hydrogen , 8 parts of oxygen or 35.5 parts by mass of chlorine.
- Eelement = atomic mass of element / valency
- Eacid = molar mass of acid / Basicity
- Ebase = molar mass of base / acidity
Note : Normality = n × molarity
For dilution : N1V1 = N2V2
For mixing solution : N1V1 + N2V2 = N3V3
If % strength and density of solution are given : N = %strength x d x 10 / EB
Numerical 1 : Calculate the mole fraction of ethanol in sample of solution containing 92% ethanol by mass?
Solution: 92 g of ethanol is present in 100g of solution
given : wB = 92g mB = 46 gmol-1 wA = 8g mA = 18gmol-1
nB = 92 /46 = 2 nA =8 /18 = 0.44
xB = nB / nA + nB = 2 / 2+0.44 = 0.82
xB = 0.82
Numerical 2 : A 1.6 M H2SO4 soln is 29% H2SO4.Calculate the density of Solution? Solution: M = %strength x d x 10 / MB

d = 1.21 gml-1
Numerical 3 : What is molarity of 20gram of NaOH dissolved in 1.5L of solution ? Solution: M = 20 / 40 x 1.5 = 0.33
Solubility
Solubility of a substance in a specific solvent is measured at the saturation concentration. It depends upon the nature of solute and solvent as well as temperature and pressure.
Solubility of Gases In Liquids
The volume of gas that can be dissolved in unit volume of liquid to form saturated solution at particular temperature and 1 atm. Pressure.
Factors Affecting Solubility of Gases in Liquid
1) Nature of solute and solvent :
A gas is highly soluble in solvent if it has
- High critical temperature
- High magnitude of attractive force between nucleus or high value of vanderwaal constant ‘a’.
- Polar solvent like water dissolves those gases which are polar in nature. Non polar solvent like benzene dissolves non-polar gas i.e. like dissolves like.
2) Effect of Temperature: Solubility of most of the gases in liquid is exothermic (because intermolecular forces between dissolved gas molecules and surrounding solvent molecules lowers the energy)
Gas + Solvent ↔ solution
According to Le – Chatelier principle , equilibrium will shift in backward direction with increase in temperature , so solubility decreases.
3) Effect of pressure : Effect of pressure on solubility of gas in liquid is explained by William Henry.
Henry’s Law
The mole fraction of gases in a solution is directly proportional to partial pressure of gas.
xgas α pgas
xgas = kpgas
pgas = xgas
pgas = KH xgas [KH = Henry’s constant]
Limitations of Henry’s Law
1) This law is applicable to gases only under ideal condition i.e under low pressure and at high temperature.
2) The molecule of gas should not dissociate or associate in solution.
3) The gases should not react with water and form compound .
NH3 + H2O ↔ NH4+(aq) + OH–(aq)
Characteristics of KH
1) KH for a gas varies with nature of solvent.
2) The value of KH for a particular gas increases with rise in temperature.
Applications of Henry’s Law
1) In Carbonated Drinks : Soft drinks and Soda water bottles are sealed with high pressure to increase the solubility of CO2 in liquid.
2)In Scuba divers equipment : Underwater pressure is high , breathing at increased pressure increases the solubility of gases in blood. When divers come towards the surface , pressure decreases . Dissolved gases are released which leads to formation of bubbles of N2 in blood . This block capillaries and creates the disease called BENDS. Thus, He gas is added to tank to reduce the painful effect.
3)High Altitude sickness (Anoxia) : At high altitude , the partial pressure of oxygen is less than that at ground level. At low pressure , solubility of oxygen in blood decreases. Low concentration of oxygen in blood causes Anoxia.
Solubility of Solids in Liquid
Solubility of solid in liquid at any temperature is defined as the maximum amount of solid in gm which can be dissolved in 100 gm of liquid to form saturated solution.
Factors affecting Solubility of Solids in Liquids
1) Nature of Solute and Solvent : Ionic and polar solute generally dissolves in polar solvent while non-polar solute are soluble in non-polar solvents.
2)Concentration of Solution : When a solute is initially added to solvent at a temperature its solubility is maximum , as concentration increases the rate of dissolution of solute decreases.
3) Temperature : If the dissolution process is endothermic , solubility should increase with rise in temperature whereas if it is exothermic solubility should decrease. Some solids do not follow regular trend : Na2SO4.10H2O called Glauber ’s salt, solubility increases on raising temp. up to 32.4oC and dissolution is endothermic . Beyond this temperature salt becomes anhydrous and dissolution is exothermic.
4)Pressure : It has negligible effect on solubility.
Solubility of Liquid in Liquid
When two liquids are mixed , their solubility or miscibility will depend upon magnitudes of attractive force between particles of two liquid.
Liquid Solution
The solution in which solvent is liquid. Solvent is always volatile. Solute may or may not be more volatile.
Vapour Pressure of Liquid Solution
Vapour pressure is the pressure exerted over the surface of liquid at equilibrium at given temperature.
Factors affecting Vapour Pressure
1) Nature of liquid : V.P of liquid α
2) Temperature : V.P of liquid α Temperature
Raoult’s Law and Vapour Pressure of Liquid Solution
The partial pressure of a volatile component present in a solution is directly proportional to the mole fraction of that component at a given temperature.
Raoult ‘s Law is also stated as (for Volatile Liquid)
In a solution , vapour pressure of a particular component at a given temperature is equal to mole fraction of that component in solution multiplied by its V.P when present in pure form.
pA = xA. pAo
pB = xB. pB o
According to Dalton’s law of partial pressure :
PS = pA + pB
ps = xA. pAo + xB. pB o
pS = (1- xB ) pAo + xB. pB o
ps = pAo + xB(pB o─ pAo)
Raoult ‘s Law ( for Non-Volatile Solute)
If solute is non-volatile and non-electrolyte , it will not contribute to total vapour pressure of solution.
PS = pA + pB [pB = 0 as solute is non volatile]
PS = pA = xA. pAo
PS / pAo = 1-xB
pAo – PS represents lowering in vapour pressure
xB = 1- PS / pAo
pAo – PS / pAo represents relative lowering in vapour pressure
xB = pAo – PS / pAo
Classification of Liquid in Liquid solution
Ideal and Non Ideal Solution
| Ideal Solution | Non Ideal Solution |
| The solution in which each component obeys Raoult ’s law under all condition of temperature and pressure. | The solution in which each component does not obey Raoult ’s law under all condition of temperature and pressure. |
| ΔH mixing = 0 (no change in heat) | ΔH mixing ≠ 0 |
| ΔV mixing = 0 | ΔV mixing ≠ 0 |
| The force of attraction between A-B is same as A-A and B-B interaction. | The force of attraction between A-B is not same as A-A and B-B interaction. |
| PS = PA + P B | PS ≠ PA + P B |
| Ex : Solution of n-hexane and n- heptane | Ex: Solution of Acetone and CS2 |
Types of Non Ideal Solution
| Solution showing +ve deviation | Solution showing –ve deviation |
| A-B interaction are weaker than those between A-A and B-B interaction. | A-B interaction are more than those between A-A and B-B interaction. |
| ΔH mixing = +ve (endothermic) | ΔH mixing = -ve (exothermic) |
| ΔV mixing = +ve (increase) | ΔV mixing = -ve (decrease) |
| Dissolution process is endothermic. | Dissolution process is exothermic. |
| PA > pº A . x A | PA < pº A . x A |
| Solubility of solute increases with rise in temperature. | Solubility of solute decreases with rise in temperature. |
| Ex: H2O + Ethanol | Ex : H2O + HNO3 |
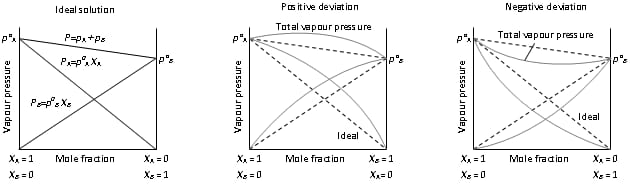
Azeotropes or Azeotropic Mixture
The liquid mixture which boils at same temperature and have same composition of constituents in liquid and vapour state is called Azeotrope OR Azeotropic mixture OR Constant boiling liquid mixture.
Characteristics
- The liquid mixture i.e both component have same boiling point at a particular temperature.
- Both components have same amount in liquid and vapour state at this temperature.
- It is not possible to separate component of liquid mixture by fractional distillation.
Types of Azeotrope
- Maximum Boiling Azeotrope: The azeotrope are formed by those liquid phase which show –ve deviation from ideal behavior because escape tendency of component is less . So azeotrope have higher boiling point than component and minimum vapour pressure. Ex : 68%HNO3 and 32% H2O by mass with b.pt of 393.5K .
- Minimum Boiling Azeotrope : The azeotrope are formed by those liquid phase which show +ve deviation from ideal behavior because escape tendency of component is high . So azeotrope have lower boiling point than component and maximum vapour pressure. Ex :Ethanol and H2O
Colligative Properties
The properties of ideal or dilute solution containing non- volatile solute which depends upon relative number of solute and solvent particles but do not depend upon their nature are called as Colligative Properties.
Relative lowering of Vapour Pressure
The vapour pressure of a solution decreases on addition of non- volatile solute to it.
As we have derived; xB = pAo – PS / pAo but this formula is applicable only when solution is dilute. Thus it is difficult to decide whether calculation should be done by assuming solution to be dilute or not .


Elevation in Boiling Point
Boiling point of the liquid is the temperature at which its vapour pressure is equal to its external pressure (1atm pressure ). Ex : Water boils at 373.15K (100oC) because at this temperature vapour pressure of water is 1.013 bar (1 atm). Note : boiling point of a solution is always higher than that of pure solvent.
Factors affecting Boiling Point
- Nature of liquid : boiling point increases with increase in intermolecular force.
- Atmospheric Pressure : b.pt increases with increase in atmospheric pressure.
When a small amount of non volatile solute is added to volatile solvent , the vapour pressure of solution will decrease. This mean solution have to be heated at high temperature . Thus, boiling point of a solution(Tb) is more than that of pure solvent (Tb0) and there will be elevation in b.pt.
ΔT b = Tb – Tºb For a small range of concentration , elevation in b.pt is directly proportional to decrease in vapour pressure.
Molal Elevation Constant or Ebullioscopy Constant
It is defined as elevation in boiling point produced when 1 mole of solute is dissolved in 1000g of the solvent.
Numerical :The boiling point of benzene is 353.23 K . When 1.8 g of non-volatile solute was dissolved in 90g of benzene boiling point is raised to 354.11 K . Calculate molar mass of solute.
Kb for benzene is 2.53 K kg mol-1 ?
Soln : ΔT b = 354.11- 353.23 = 0.88K
ΔT b = kb x m MB = 58 gmol-1
Depression in freezing Point
Freezing Point of a substance is defined as the temperature at which the vapour pressure of substance its liquid phase is equals to its vapour pressure in solid phase.

ΔT f = Tºf – Tf
Addition of a non- volatile solute to the solvent decreases its vapour pressure so the equilibrium achieved between solid and liquid phase at a lower temperature hence freezing point is depressed.
ΔT f = Kf × m
Note: derivation is same as that of elevation in freezing point.
- Antifreeze is a liquid which is added to another liquid to prevent its freezing or depress its freezing point.
Osmotic Pressure and Osmosis
The spontaneous flow of solvent molecules through a semi-permeable membrane from pure solvent to solution OR from dilute solution to concentrated solution is called Osmosis.
Semi – Permeable Membrane
A membrane having very small pores or sieves that permits only the movement of solvent molecules but not big size molecules i.e solute molecules is called Semi- permeable membrane (SPM).
Types of SPM
- Natural SPM
- Artificial SPM
Osmotic Pressure
The external pressure that has to be applied on the solution to prevent the osmosis of the solvent into it through a semi – permeable membrane. It is indicated by π.
According to Boyle – Van’t Hoff Law : Π ∝ C at constant temperature According to Gay-lussac’s Van’t Hoff Law : Π ∝ T
Thus , Π = RCT
Limitation of Osmometric Method in Molecular Mass Determination
- Molecular mass of only non-volatile solute can be determined.
- Solution of unknown compound must be very dilute.
- Solute molecule should not undergo dissociation or association.
Note:
- Isotonic Solution
Such solution which have same osmotic pressure at same temperature are called isotonic or Iso-osmotic solution.
- Hypertonic Solution
A solution having higher osmotic pressure relative to other solution are called hypertonic solution
- Hypotonic Solution
A solution having lower osmotic pressure relative to other solution are called hypotonic solution.
Exo-Osmosis and Endo-Osmosis
The cell wall of plant acts as semi permeable membrane.
- When plant cell is placed in hypotonic solution then water molecules will enter into the cell and cell will swell up . This is called endo-osmosis. Ex : Swelling or rupturing of blood cell.
- When plant cell is placed in hypertonic solution then water molecules will move out of the cell and cell will shrink . This is called Exo-osmosis.
Note: RBCS are isotonic with 0.9% aq. Solution of NaCl.
Note: Pickeled vegetables appear shriveled because they lose water to hypertonic medium in which they are preserved.
Reverse Osmosis
(From Higher Concentration to Lower Concentration)
The direction of osmosis can be reversed if external pressure is higher than osmotic pressure. Generally, SPM used is cellulose acetate. For e.g. Desalination of sea water.
Numerical : 10g of substance were dissolved in water and solution was made up to 250 cm3 . The osmotic pressure of the solution was found to be 8 × 105 Nm-2 at 288K. Find the molar mass of solute ?
Soln : MB = 119.7 gmol-1
Abnormal Colligative properties or Abnormal Molecular Mass
Accurate value of molecular mass or colligative property can be obtained when solute are non-electrolyte but when solute is electrolyte i.e. Acid, Base and Salt , they undergo association or dissociation leading to change in no of particles. Hence, Colligative properties are also changed.
Abnormal value of α No of particles after
colligative property association or dissociation
Van’t Hoff Factor : It predicts the nature of solute in solution.
i = observed colligative property / normal colligative property
i = normal molecular mass / Abnormal molecular mass
Note :
- If i=1 solute behaves normally in solution
- If i>1 solute undergoes dissociation in solution
- If i<1 solute undergoes association in solution
When solute is electrolyte , then colligative properties are as follow;
- Relative lowering of vapour pressure : pAo – PS / pAo = i xB
- Elevation in Boiling point : ΔT b = i(Kb × m)
- Depression in freezing point : ΔT f = i( Kf × m )
- Osmotic Pressure : ∏ = i CRT
Degree of Dissociation or Association
It is the fraction of total substance that undergoes dissociation or association. α = no of moles dissociated or associated / total no of moles taken.
Relation between α and i
In dissociation : α = i-1 / n-1
In association : α = i-1 / (1/n) -1
————————
# Solutions Chemistry Class 12
# Solutions Chemistry Class 12 Notes
# Solutions Chemistry Class 12 Solutions
Do share the post if you liked Solutions Chemistry Class 12. For more updates, keep logging on BrainyLads




Wonderful…… extremely useful notes !
Thanks a lot Ma’am