P Block Elements Notes | Group 15 | Chapter 7 | Chemistry |
Table of Contents
P Block Elements Notes | Part I | Group 15 | Chapter 7 | Chemistry | CBSE |
P Block Elements Part 1
Group 15
- The elements of Nitrogen family i.e. group 15 of the periodic table are: nitrogen (N), phosphorous (P), arsenic (As), antimony (Sb) and bismuth (Bi).
- Collectively, group 15 elements are called pnicogens and their compounds are called pniconides. (It means suffocation producing elements)
| Symbol | Element | Atomic No | Electronic configuration | Group No | Period No |
| N | Nitrogen | 7 | [He] 2s2 2p3 | 15 | 2 |
| P | Phosphorous | 15 | [Ne] 3s2 3p3 | 15 | 3 |
| As | Arsenic | 33 | [Ar] 4s2 4p3 | 15 | 4 |
| Sb | Antimony | 51 | [Kr] 4d10 5s2 5p3 | 15 | 5 |
| Bi | Bismuth | 83 | [Xe] 4f14 5d10 6s2 6p3 | 15 | 6 |
- Nitrogen and Phosphorous are non metals
- Arsenic and Antimony are metalloids
- Bismuth is a moderate metal
Atomic Properties
Atomic size or Radii
a)The atomic and ionic radii of group 15 are smaller than corresponding elements of group 14 because on moving from left to right Zeff increases as new electrons are added in same subshell and hence electrons are more strongly attracted towards the nucleus .
b) On moving down the group , ionic and covalent radii increases due to increase in number of shells .
Ionization Enthalpy
The amount of energy which is required to remove an electron from isolated gaseous atom of an element .
Factors affecting Ionization Enthalpy are as follows :
I.E ∝ Effective Nuclear Charge
I.E ∝ 1 / Atomic size
I.E ∝ 1 / Shielding Effect
Along Period : The I.E of group 15 are greater than group 14 because elements of group 15 has smaller atomic size than group 14 due to increase in effective nuclear charge along period .
Along Group : I.E of group 15 decreases down the group due to increase in number of shells .
Electronegativity
The tendency of atom of an element to attract the shared pair electron towards itself .
Factors affecting Electronegativity are as follows :
Electronegativity ∝ Effective Nuclear Charge
Electronegativity ∝ 1 / Atomic size
Electronegativity of group 15 elements is higher than group 14 because group 15 elements have stable electronic configuration .
Electronegativity of group 15 decreases down the group due to increase in atomic size down the group.
Physical Properties
Melting and Boiling Points
Melting point increases from N to As and after that it starts to decrease because from N to As their melting point depends on magnitude of vanderwaal force.They form five covalent bond so melting point increases upto Arsenic . After , Arsenic the tendency of making 5 covalent bond decreases due to inert pair effect. So Sb and Bi form 3 covalent bonds instead of 5 .
The boiling point increases regularly with increase in atomic number as we move down the group.
Catenation (Self Linking Property)
Question : Nitrogen exist as diatomic gas and phosphorous exist as P4. Why ?
Answer : Nitrogen exist as diatomic gas because due to small size of nitrogen atom it has strong tendency to form p—p multiple bonding . Other element exist as octahedral molecule because they are not capable of forming p p multiple bonds as their atomic orbital are so large and diffuse that they cannot have effective overlapping.
Question : Why N2 exist is less reactive at room temperature?
Answer : N2 molecule contains N≡N , this bond is very stable and require high bond dissociation enthalpy . So it is less reactive at room temperature .
Question : Why N2 exist as gas while P , As and Sb exists as solid ?
Answer : The N–N single bond is weaker than single P– P bond because of high inter-electronic repulsion of non-bonding electrons as a result of small bond length formed . So N2 exist as gas and other exist as solid and due to this N2 also show less catenation.
Metallic Character
The element of group 15 are less metallic than corresponding elements of group 14 due to increased effective nuclear charge and electronegativity of group 14 . However, on moving down the group , the metallic character increases because down the group electronegativity decreases. As a result valance electrons are lost more readily and hence the metallic character increases.
Chemical Properties
Oxidation States
Negative Oxidation State :
- The tendency of formation of M 3- of element decreases as we move down the group from N to Bi due to gradual decrease in their electronegativity and ionization enthalpy .
- N and P accept three electrons and form Nitride and Phosphide ion respectively while other elements do not form since N and P have high Zeff and small size , so electron accepting tendency of three electron is high .
- Besides -3 , N and P also show -2 oxidation state in Hydrazine (NH2NH2) and diphosphine (P2H4) respectively.
Positive Oxidation State :
- All the element of group 15 show positive oxidation state of +3 and +5.
- On moving down the group , stability of + 5 oxidation state decreases while that of +3 oxidation state increases due to inert pair effect.
Note : Nitrogen because of its small size , high electronegativity and tendency to form pπ —pπ multiple bonds , show all oxidation state from -3 to +5.
Maximum Covalency
- Nitrogen show maximum covalency of 4 due to absence of d orbital in its valence shell.
- Phosphorous , Arsenixc and Antimony element have empty d orbital and can utilise all their orbital to exhibit covalency of 5 or 6 .
- Though , Bismuth has vacant d orbital yet it does not show covalency 5 due to inert pair effect .
Disproportionation
- All the oxidation state of Nitrogen from +1 to +4 disproportionate in acidic medium.
3HNO2 → HNO3 + H2O + 2NO
- Similarily , In Phosphorous nearly all oxidation state from -3 to +5 show disproportionation both in acidic and basic media .
4H3PO3 → 3H3PO4 + PH3
Trends in Chemical Reactivity
Reactivity towards Hydrogen (Formation of Hydrides )
All elements of group 15 form volatile trihalides of formula EH3 where E= N, P, As, Sb, Bi
NH3 = Ammonia PH3 = Phosphine AsH3 = Arsine SbH3 = Stibine BiH3 = Bismuthine
Preparation of Hydrides
By hydrolysis of binary metal compounds :
- Mg3N2 (Magnesium nitride) + 6H2O → 3Mg(OH)2 + 2NH3 (Ammonia)
- Zn3As2 (Zinc arsenide) +6HCl → 3ZnCl2 + 2AsH3 (Arsine)
- Ca3P2 (calcium phosphide) + 6H2O → 3Ca(OH)2 + 2PH3 (Phosphine)
By reduction of trichlorides :
ECl3 +3LiAlH4 → EH3 + 3LiCl + 3 AlH3 (E = N, P, As, Sb)
Structure
All the hydrides of group15 elements have pyramidal structure. All these elements undergo sp3 hybridization.
Question : Why HNH angle in NH3 is higher than HPH (PH3) ?
Answer : Bond angle ∝ Electronegativity and size of central atom
In NH3 , N atom is more electronegative and has small size. Hence, it attracts bond pair towards it and these bond pair repel the lone pair and bond angle starts to increases. While from N →Bi , electronegativity decreases and size increases. Hence, bond angle decreases.
Properties of Hydrides
Boiling points
The boiling points increases regularly as we move from PH3 to BiH3 since due to increase in molecular mass , van der waals force of attraction also increases resulting in increased boiling point.
Question : Why boiling point of NH3 is higher than PH3 and AsH3?
Answer: The abnormally high boiling point of NH3 is due to intermolecular hydrogen bonding.
Question: Why NH3 has less boiling point than SbH3 and BiH3?
Answer: This is because high van der waals force of attraction in SbH3 and BiH3 dominates over hydrogen bonding in NH3.
The boiling point of hydrides of group 15 elements follows the order:
PH3 < AsH3 < NH3 < SbH3 < BiH3
Melting Points
Because of intermolecular H-Bonding, NH3 has the highest melting point.
Other hydrides of this group do not form H-Bonding and hence their melting point are lower than that of NH3.
Melting point increases regularly from PH3 to SbH3 due to increase in molecular mass, van der waals force of attraction also increases.
The melting points of hydrides of group15 elements follows the sequence: PH3 < AsH3 < SbH3 < NH3
Solubility
NH3 forms H-bonds with water while other hydrides do not. Therefore, NH3 is soluble in H2O while other hydrides are insoluble in H2O.
Thermal Stability
As we move down the group the size of the central atom increases and hence its tendency to form covalent bond with comparatively small H atom decreases. M—H bond strength also decreases and hence thermal stability also decreases as we move from NH3 to BiH3.
Thermal stability of hydrides of group15 element decreases in the order:
NH3 > PH3 > AsH3 > SbH3 > BiH3
Reducing Character
As we move from NH3 to BiH3, the thermal stability of hydrides decreases. Thus, their tendency to liberate hydrogen increases and hence reducing character increases from NH3 to BiH3.
Reducing character of hydrides of group15 element increases in order:
NH3 < PH3 < AsH3 < SbH3 < BiH3
Basic Nature
All the hydrides of group15 possess a lone pair of electron on the central atom and thus behave as Lewis base.
Basic nature of hydrides of group15 follows the sequence : NH3 > PH3 > AsH3 > SbH3 > BiH3
As the size of central atom increases, the lone pair of electron occupies a large volume. In other words, electron density on central atom decreases and consequently its tendency to donate a lone pair of electron decreases and hence basic strength decreases as we move from NH3 to BiH3.
Reactivity towards Halogen (Formation of Halides)
A)Trihalides
All elements of group15 form trihalides with general formula EX3
Structure
Trihalides have pyramindal structure, i.e., central atom is sp3 hybridised.
The bond angle of trihalides of group15 decreases in order: PF3 < PCl3 < PBr3 < PI3
This trend is due to reason that as electronegativity of halogen increases, the bond pair move away from the central P atom. As a result, bond pair – bond pair repulsion decreases and hence also bond angle also decreases.
Properties
Question: Discuss the stability of trihalides of Nitrogen ?
Answer: NF3 is only stable trihalide of nitrogen due to strong bond strength of N—F bond since both have comparatively small size.
The instability of NCl3, NBr3, NI3 is because of the weakness of N—X bond due to large size difference in size of N and X atom.
Question: Why BiF3 is predominantly ionic?
Answer: In BiF3 : F- →smaller anion → least polarisability
Bi + → larger cation → least polarising power
These both factors account for least covalent nature. So it is pre dominantly ionic.
Question: Why trihalides of P, As and Sb behave as Lewis acids?
Answer : They accept a lone pair of electron due to presence of d orbital and thus behave as lewis acids.
The Lewis acid strength decreases in order : PCl3 > AsCl3 > SbCl3 and PF3 > PCl3 > PBr3 > PI3
Question: Why trihalides of Nitrogen behave as Lewis base?
Answer : They act as lewis base due to presence of lone pair and absence of d orbital.
Question: Why NF3 is not a Lewis base?
Answer: NF3 has a little tendency to donate a pair of electron because of the high electronegativity of F.
The lewis base strength of other trihalides increases as electronegativity of halogen decreases.
The Lewis base strength increases in order : NF3 < NCl3 < NBr3 < NI3
Question: Why Nitrogen triiodide ammoniate is stable only in the moist state?
Answer: This is because in dry state , it explodes with noise when struck liberating vapours of I2. Thus, it is a mild and harmless explosive. 8NI3.NH3 → 5N2 + 9I2 + 6NH4I
B)Pentahalides
P, As and Sb form pentahalides of the general formula EX5 due to presence of vacant d orbitain their respective valence shells.
Question : Why N does not form pentahalides?
Answer: This is due to absence of d orbital in its valence shell.
Question: Why pentahalides are thermally less stable than trihalides?
Answer : This is because of the following two reasons:
- As we move down the group, stability of +3 oxidation state increases while that of +5 oxidation state decreases due to inert pair effect.
- As the size of halogen increases from F to I, the strength of the phosphorous- halogen bond decreases and steric hindrance increases.
Question: Why PCl5 behave as good chlorinating agent?
Answer: As pentahalides are thermally less stable than the corresponding trihalides. Thus, PCl5 is decomposed and liberates Cl2. Hence it acts as good chlorinating agent.
PCl5 → PCl3 + Cl2
Question: Why PF5 does not undergo hydrolysis?
Answer: This is because P—F bond is stronger than P—O bond.
Question: Why pentachloride, pentabromide and pentaiodide of Bi does not exist?
Answer: This is probably due to the strongly oxidising properties of Bi5+ due to inert pair effect.
Question: Why all pentahalides behave as lewis acid?
Answer: All the pentahalides acts as lewis acid due to presence of vacant d orbital, the central atom can accept a pair of electrons thereby expanding its coordination number to 6, i.e., MX5 + X– → [MX6]– . As a result, the hybridization of the central atom changes from sp3d to sp3d2.
Question: Why pentahalides are less covalent than trihalides?
Answer: In pentahalides, the oxidation state of central atom is high and thus its charge density and polarising power is also high. This results in greater covalent nature.
Question: Bromides are more covalent than trihalides. Explain?
Answer: Size of bromide is greater than than chlorides. So it has more polarisability and hence it has more covalent nature.
Question: Discuss the bond nature of pentahalides of P?
Answer: PCl5 is covalent in vapour state but exist as [PCl4]+[PCl6]– in the crystalline state; the ions have tetrahedral and octahedral structure respectively. In the solid state PBr5 exists as [PBr4]+ Br– while PI5 exists as [PI4]+ and I– in solution.
Reactivity towards Oxygen (Formation of Oxides)
Nitrogen has a strong tendency to form pπ—pπ multiple bond between Nitrogen and oxygen, whereas other elements of this group do not form.
| Oxidation State of Element | Oxides of Nitrogen | Oxides of Phosphorous | Oxides of Arsenic | Oxides of Antimony | Oxides of Bismuth |
| +1 | N2O
Nitrous oxide |
— | — | — | — |
| +2 | NO
Nitric oxide |
— | — | — | — |
| +3 | N2O3
Dinitrogen trioxide |
P4O6
Phosphorous trioxide |
As4O6
Arsenic trioxide |
Sb4O6
Antimony trioxide |
Bi2O3
Bismuth trioxide |
| +4 | N2O4
Dinitrogen tetraoxide |
P4O8
Phosphorous tetraoxide |
— | — | — |
| +5 | N2O5
Dinitrogen pentoxide |
P4O10
Phosphorous pentoxide |
As4O10
Arsenic pentoxide |
Sb4O10
Antimony pentoxide |
— |
Oxides of non metal are acidic, those of metalloids are amphoteric while that of metals are basic. Further, greater the electronegativity of the element , more acidic is the oxide. Among the oxides of the same element, higher the oxidation state of the element, more is its acidic strength.
Acidic strength of oxides of nitrogen increases in the order: N2O < NO < N2O3 < N2O4 < N2O5
Acidic strength of trioxides follows the order: N2O3 > P4O6 > As4O6 > Sb4O6
Acidic strength of pentoxides follows the order: N2O5 > P4O10 > As4O10 > Sb4O10
Dinitrogen
Nitrogen exists as diatomic gas in the elemental state. Thus, it is called dinitrogen.
Preparation
By thermal decomposition of ammonium nitrite: NH4Cl + NaNO2 → N2 + 2H2O + NaCl
By thermal decomposition of ammonium dichromate: (NH4)2Cr2O7 → N2 +4H2O + Cr2O3
By thermal decomposition of sodium or barium azide:
2NaN3 (sodium azide) → 2Na + 3N2 Ba(N3)2 (barium azide)→ Ba + 3N2
Physical Properties
- Dinitrogen is a colourless, tasteless, non-toxic gas.
- It has two stable isotopes: 14N and 15N
- It is very slighly soluble in water.
- It has low freezing (63.2K) and boiling point (77.2K).
- It is adsorbed by activated charcoal.
Question: Why is N2 inert at room temperature?
Answer: N2 contains a triple bond. This bond is very stable due to high bond dissociation enthalpy. Thus, it is inert at room temperature.
Chemical Properties
Action of active metal: Many active metal when heated at high temperatures, keep on burning in an atmosphere of dinitrogen forming their ionic nitrides.
6Li + N2 → 2Li3N (Lithium nitride)
2Al + N2 → 2AlN (Aluminium nitride)
3Mg + N2 → Mg3N2 (Magnesium nitride)
3Ca + N2 → Ca3N2 (Calcium nitride)
Action of dihydrogen (Haber’s process) : When a mixture of dinitrogen and dihydrogen is heated at 700K under 200 atm, in presence of iron oxide as catalyst and molybdenum as promoter, ammonia is formed.
N2 + 3H2 ↔ 2 NH3
Question: What do you mean by active nitrogen?
Answer: It is prepared by passing an electric spark through N2 gas at low pressure. It forms active nitrogen and this process is associated with yellow- pink after glow. It is very reactive and reacts with no of elements and break many stable molecules.
HC ≡ CH (Acetylene) + 2N (active nitrogen) → H≡C—C≡N (Cyanogen) +H2
Ammonia
Preparation
By heating ammonium salt with stronger base:
(NH4)2SO4 (ammonium sulphate) + 2NaOH → 2NH3 + 2H2O + Na2SO4
2NH4Cl + Ca(OH)2 (slaked lime) → 2NH3 + 2H2O + CaCl2
NH4Cl + KOH → NH3 +H2O +KCl
By the action of water on metal nitrides:
Mg3N2 (magnesium nitride) +6H2O → 3Mg(OH)2 +2NH3
AlN (aluminium nitride) + 3H2O → Al(OH)3 + NH3
Physical Properties
- Ammonia is colourless gas with characteristics pungent smell called the ammonical smell.
- It is lighter than air and extremely soluble in water.
- When vapourised, liquid ammonia causes intense cooling.
Structure
Nitrogen atom in ammonia is sp3 hybridised. NH3 molecule has pyramidal geometry.
Chemical Properties
It is highly soluble in water. NH3 + H2O ↔ NH4+ + OH–
Question: How is NH3 used to detect metals?
Answer: It forms complex compound with metals.
Cu2+ + 4NH3 ↔ [Cu(NH3)4]2+ (deep blue colour)
Ag+ (colourless) + Cl– → AgCl (white ppt) AgCl + 2NH3 → [Ag (NH3)2]Cl (colourless)
Oxidation:
- 4NH3 + 3Ca(OCl)2 → 2N2 + 3CaCl2 +6H2O
- 8 NH3 +3Br2 → N2 + 6NH4Br
- 4NH3 +5O2 → 4NO + 6H2O
Nitric Acid
Preparation
In laboratory
NaNO3 +H2SO4 → NaHSO4 +HNO3
On large scale it is prepared by Ostwald’s process
- 4NH3 +5O2 → 4NO + 6H2O
- 2NO + O2 → 2NO2
- 3NO2 + H2O → 2HNO3 + NO
Physical Properties
- It is colourless fuming liquid with pungent odour.
- Pure nitric acid freezes at 231.4K and boils at 355.6K
- It acquires a yellowish brown colour in presence of light.
Chemical Properties
Question : Why HNO3 acts as strong oxidising agent?
Answer: It is a very strong oxidising agent since it readily gives nascent oxygen.
2HNO3(conc.) → H2O + 2NO2 + [O]
2HNO3 (dil.) → H2O + 2NO +3[O]
Question: Write some reactions which shows concentrated nitric oxide also oxidises non metal and their compounds?
Answer :
I2 +10HNO3 → 2HIO3 +10NO2 +4H2O
C + 4HNO3 → CO2 +2H2O + 4NO2
S8 +48HNO3 → 8H2SO4 + 48NO2 +16H2O
P4 +20HNO3 → 4H3PO4 +20NO2 +4H2O
Question : What do you mean by passivity of metals in HNO3?
Answer: When dipped in conc. HNO3, metals like iron, chromium, nickel and aluminium loose their normal activity and become passive. The passivity of these metals is due to formation of thin protective layer of the metal oxide on surface of the metal which prevents further action. Example:
3Fe + 8HNO3 → FeO.Fe2O3 + 8NO2 + 4H2O
Question: Explain Ring test for nitrate ion?
Answer: The brown ring test depends upon the ability of Fe2+ ions to reduce nitrate ions to nitric oxide which then reacts with Fe2+ ions to form brown coloured complex.
NO3 +3Fe2+ +4H+ → NO + 3Fe2+ +2H2O
[Fe(H2O)6]2+ + NO → [Fe (H2O)5 (NO)]2+ (brown) +H2O
Allotropic Forms of Phosphorous
1)White or Yellow Phosphorous
Preparation : 2Ca3(PO4)2 + 6SiO2 → 6CaSiO3 +P4O10
P4O10 +10C → P4 + 10CO
Properties:
- On exposure to light, white phosphorous turns yellow, therefore, it is called yellow phosphorous.
- It is soft, translucent waxy white solid with garlic odour.
- It is very poisonous. Persons working with white phosphorous develop a disease known as Phossy jaw in which jaw bones decay.
- It is insoluble in water, but soluble in organic solvents such as CS2 , alcohol and ether.
- When it burns in air, it produces white fumes of phosphorous pentoxide P4 + 5O2 → P4O10
- Because of its very low ignition temperature, it is always kept under water.
- It is very reactive and readily catches fire in air with a greenish glow which is visible in dark.
2)Red Phosphorous
Preparation: It is obtained by heating white phosphorous at 573K in an inert atmosphere for several hours.
Properties:
- It is hard crystalline odourless solid with iron grey lusture.
- It is non- poisonous in nature.
- It is insoluble in water as well as in organic solvent.
- It is denser than white phosphorous and is a bad conductor of electricity.
- Being polymeric in nature, it is less reactive than white phosphorous.
- Its ignition temperature (543K) is much higher than that of white phosphorous (303K). Thus, it does not catch fire easily.
3)Black Phosphorous
It has two forms : α-black phosphorous and β- black phosphorous.
Preparation: α-Black phosphorous is formed when red phosphorous is heated in a sealed tube at 803K while β- black phosphorous is prepared by heating white phosphorous at 473Kunder a sealed high pressure in an inert temperature.
Properties:
- It is highly polymerized form of phosphorous and has black metallic lusture.
- It has sharp melting point of 860K.
- Like graphite, it is fairly good conductor of electricity.
- It is thermodynamically most stable allotrope of phosphorous and does not burn in air even upto 673K.
Phosphine, PH3
Preparation
From Metal Phosphide : Ca3P2 (calcium phosphide) +6H2O → 3Ca(OH)2 + 2PH3 ↑
Ca3P2 +6HCl → 3CaCl2 +2PH3↑
Laboratory method:
P4 +3NaOH +3H2O → 3NaH2PO2 (Sod. Hypophosphite) + PH3 ↑
Structure
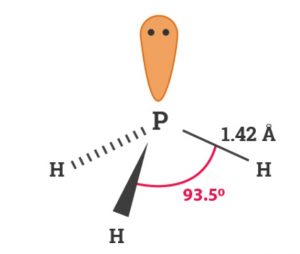
P in PH3 involves sp3 hybridisation. It has pyramidal structure.
Physical Properties
- It is colourless gas possessing unpleasant odour similar to that of rotten fish smell and is highly poisonous.
- It is slightly soluble in water. However , it is more soluble in organic solvents.
- It is little heavier than air.
Chemical Properties
Basic Nature: It is feebly basic and form salt with mineral acids under anhydrous conditions.
PH3 + HX → PH4+X– (Phosphonium halide)
Reaction through metallic salt solution : When it is bubbled through aqueous solution of copper and mercury salts, precipitates of corresponding phosphides are formed.
3CuSO4 +2PH3 → Cu3P2 ↓ (Copper phosphide) + 3H2SO4
3HgCl2 + 2PH3 → Hg3P2 ↓ (Mercuric phosphide) + 6HCl
Decomposition : The solution of phosphine in water decomposes in presence of light giving red phosphorous and H2.
4PH3 → P4 (Red phosphorous) + 6H2
Phosphorous Halides
A) Phosphorous Trichloride
Preparation
P4 +6Cl2 → 4PCl3 P4 +8SOCl2 → 4PCl3 +4SO2 +2S2Cl2
Physical Properties
It is colourless pungent smelling liquid which boils at 347K and solidifies at 161K. It fumes in moist air.
Chemical Properties
Action of water: It reacts violently with water to produce phosphorous acid and hypochloric acid.
PCl3 + 3 H2O → H3PO3 +3HCl
It acts as reducing agent:
PCl3 + SO3 → POCl3 + SO2
PCl3 + SO2Cl2 → PCl5 + SO2
Action of organic compounds
3CH3CH2COOH + PCl3 → 3CH3COCl + H3PO3
3C2H5OH + PCl3 → 3C2H5Cl + H3PO3
B)Phosphorous Pentachloride
Preparation
It is prepared by the reaction of white phosphorous with excess of dry chlorine or by action of dry chlorine on phosphorous trichloride.
P4 +10Cl2 → 4PCl5 PCl3 +Cl2 → PCl5
Physical Properties
It is a pale yellow crystalline solid with characteristic pungent smell. In solid state it exists as ionic solid [PCl4]+[PCl6]– in which the cation is tetrahedral while the anion is octahedral.
Chemical Properties
Dissociation: When heated it sublimes but decomposes on stronger heating. PCl5 ↔ PCl3 +Cl2
Reaction with compound containing hydroxyl group:
CH3COOH (Acetic acid) + PCl5 → CH3COCl (Acetyl chloride) + POCl3 + HCl
CH3CH2OH (Ethyl alcohol) + PCl5 → CH3CH2Cl (Ethyl chloride) + POCl3 + HCl
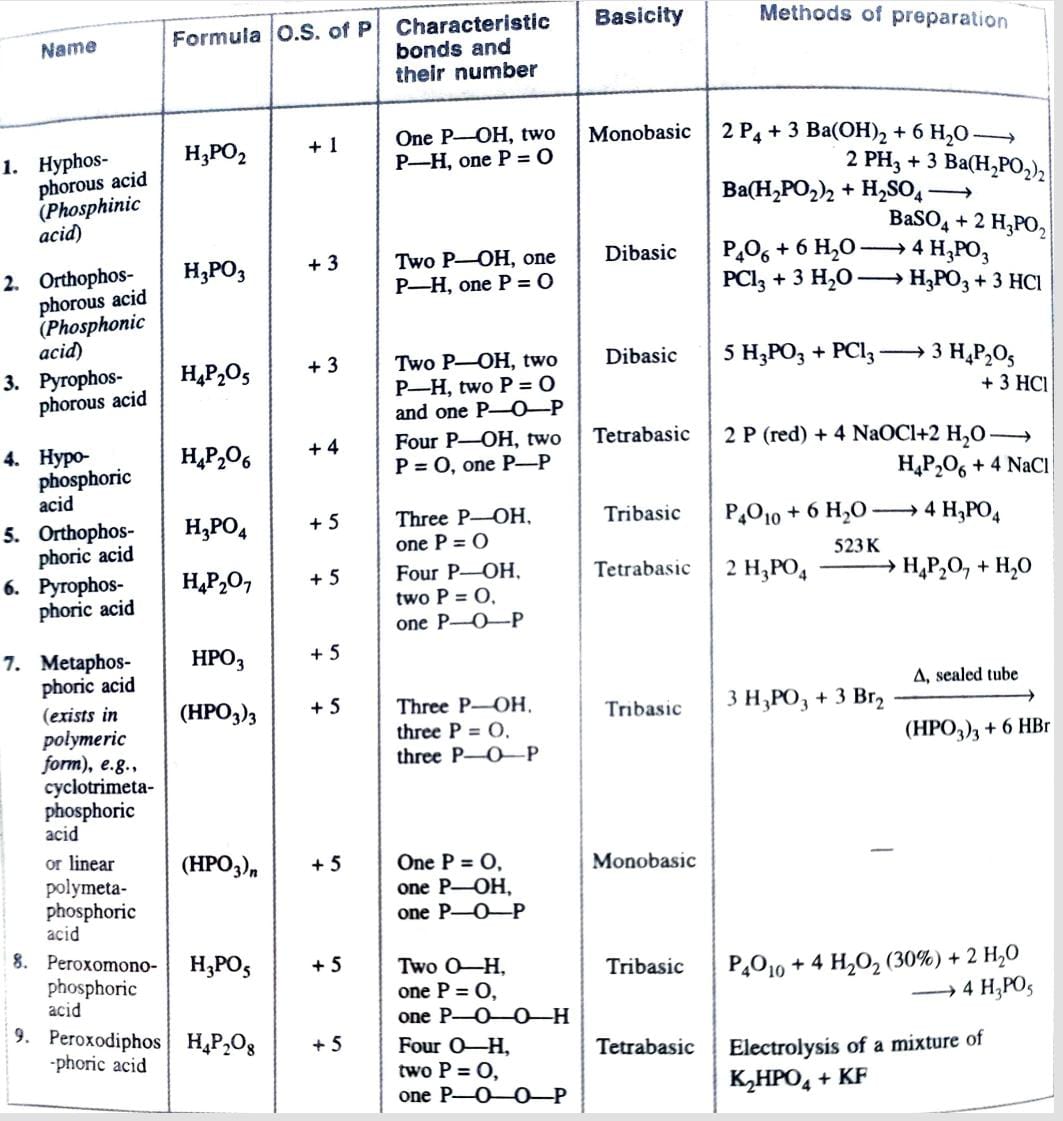
Oxoacids of Phosphorous
Structure of Oxoacids of Phosphorous
# P Block Elements
# P Block Elements Notes
# P Block Elements Part 1
# P Block Elements Part 1 Notes
# P Block Elements Part 1 Solutions
Do share the post if you liked P Block Elements Notes. For more updates, keep logging on BrainyLads