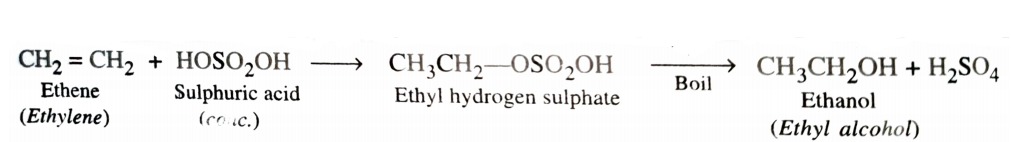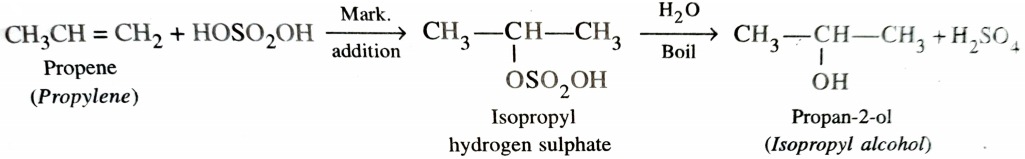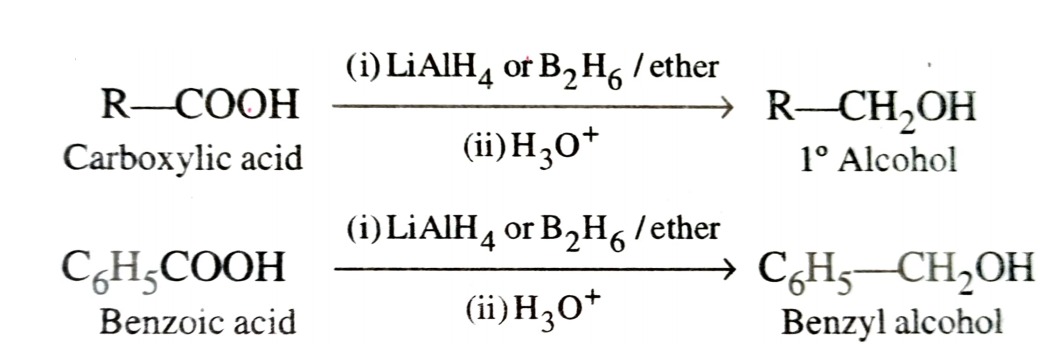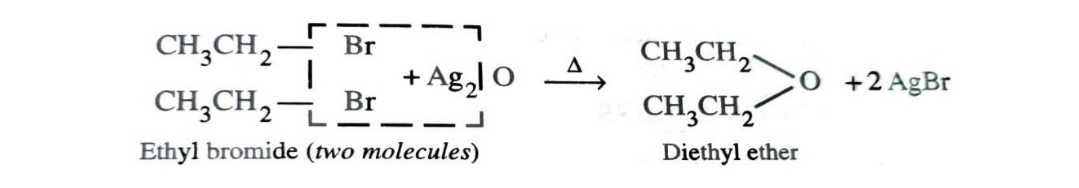Alcohols Phenols and Ethers Class 12 | Chapter 11 | Chemistry | CBSE |
Table of Contents
Alcohols Phenols and Ethers Class 12 | Chapter 11 | Chemistry | CBSE |
Alcohols, Phenols and Ethers
When a hydrogen atom in a hydrocarbon (aliphatic or aromatic) is replaced by one or more – OH group, the compound is known as alcohols or phenols. In other words, hydroxy derivatives of aliphatic hydrocarbon are called alcohols while hydroxy derivatives of benzene are called phenols.
Nomenclature of Alcohols
| Structural Formula | Common Name | IUPAC Name |
| CH3-OH | Methyl alcohol | Methanol |
| CH3-CH2-OH | Ethyl alcohol | Ethanol |
| CH3-CH2-CH2-OH | n-Propyl alcohol | Propan-1-ol |
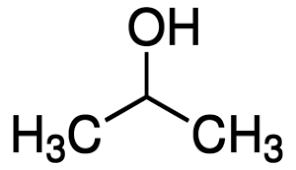 |
Isopropyl alcohol | Propan-2-ol |
| CH3-CH2-CH2-CH2-OH | n-Butyl alcohol | Butan-1-ol |
| sec-Butyl alcohol | Butan-2-ol | |
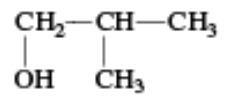 |
Isobutyl alcohol | 2-Methylpropan-1-ol |
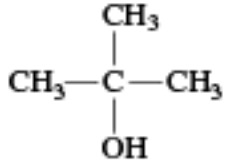 |
tert-Butyl alcohol | 2-methylpropan-2-ol |
| CH2=CH-OH | Vinyl alcohol | Eth-1-en-1-ol |
| CH2=CH-CH2-OH | Allyl alcohol | Prop-2-en-1-ol |
| HO-CH2-CH2-OH | Ethylene glycol | Ethane-1,2-diol |
| HO-CH2-CH2-CH2-OH | Trimethylene glycol | Propane-1,3-diol |
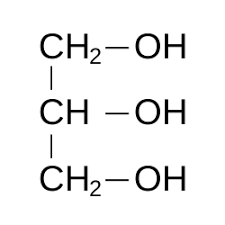 |
Glycerol | Propane-1,2,3-triol |
Nomenclature of Phenols
| Structural Formula | Common Name | IUPAC Name |
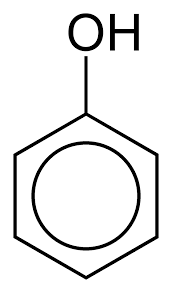 |
Phenol | Phenol |
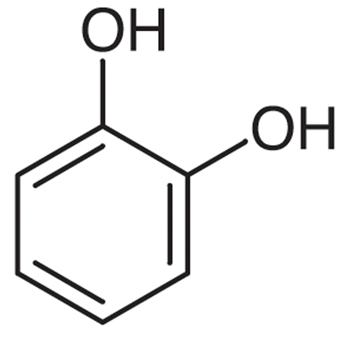 |
Catechol | Benzene-1,2-diol |
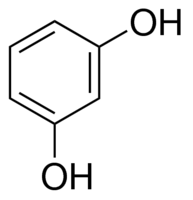 |
Resorcinol | Benzene-1,3-diol |
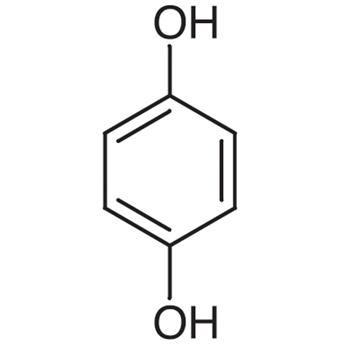 |
Quinol | Benzene-1,4-diol |
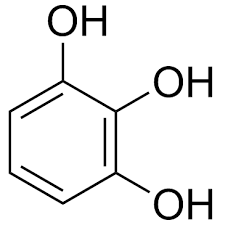 |
Pyrogallol | Benzene-1,2,3-triol |
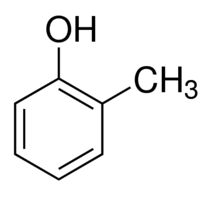 |
o-Cresol | 2-Methylphenol |
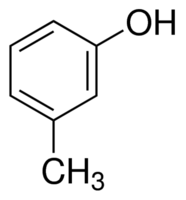 |
m-Cresol | 3-Methylphenol |
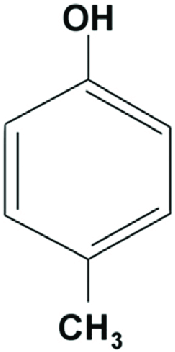 |
p-Cresol | 4-Methylphenol |
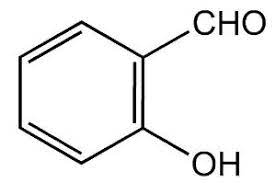 |
Salicyladehyde | 2-Hydroxybenzaldehyde |
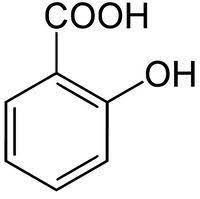 |
Salicylic acid | 2-Hydroxybenzoic acid |
Nomenclature of Ethers
| Structural Formula | Common Name | IUPAC Name |
| CH3-O-CH3 | Dimethyl ether | Methoxymethane |
| C2H5-O- C2H5 | Diethyl ether | Ethoxyethane |
| CH3-O-CH2-CH2-CH3 | Methyl n-propyl ether | 1-Methoxypropane |
| C6H5-O- CH3 | Methylphenyl ether (Anisole) | Methoxybenzene |
| C6H5-O-CH2-CH3 | Ethylphenyl ether | Ethoxybenzene |
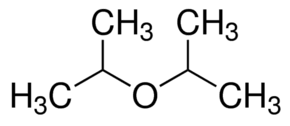 |
Di-isopropyl ether | 2-(2-Propoxy)propane |
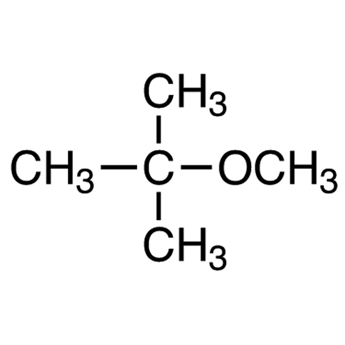 |
tert-Butyl methyl ether | 2-Methoxy-2-methylpropane |
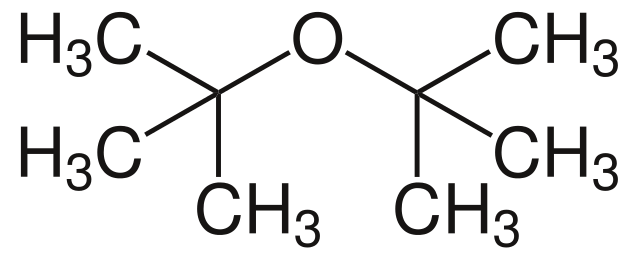 |
Di-tert-butyl ether | 2-(2-Methyl-2-propoxy)methylpropane |
| C6H5-O- C6H5 | Diphenyl ether | Phenoxybenzene |
Preparation of Alcohol
1) From Haloalkanes
When haloalkanes are heated with aqueous sodium or potassium hydroxide or moist silver oxide , they undergo hydrolysis to produce alcohols.
RX (Haloalkane) + KOH (aq) → R—OH (Alcohol) + KX
CH3CH2-Br (Bromoethane) + KOH (aq) → CH3CH2-OH (Ethanol) + KBr
CH3I (Iodomethane) + AgOH (aq) → CH3OH (Methanol) + AgI
2) From Alkenes
a) By Acid Catalysed Hydration
Hydration means addition of a molecule of water. Alkenes react with water in presence of acid as catalyst to form alcohols. This hydration can be carried out either directly or indirectly . In indirect process , alkenes are passed through concentrated H2SO4 to form alkyl halogen sulphates . These upon hydrolysis with boiling water give alcohol .
In case of unsymmetrical alkenes , the addition of H2SO4 takes place in accordance with Markovnikov ‘s rule.
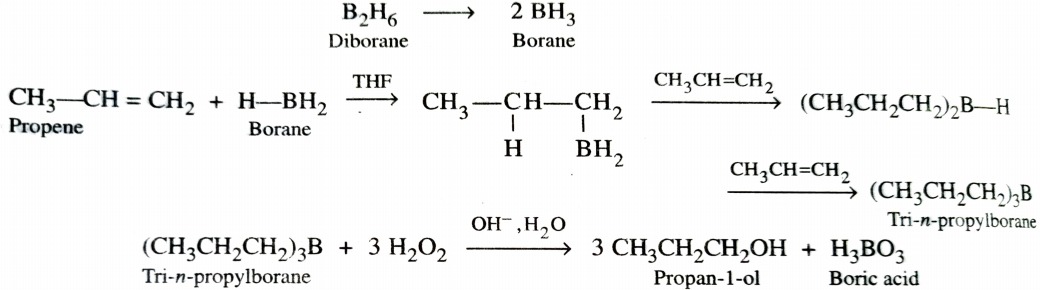
b) Hydroboration Oxidation Reaction
Diborane (B2H6) is an electron deficient molecule . Therefore , it acts as an electrophile and reacts with alkenes to form trialkylboranes which upon subsequent oxidation with alkaline H2O2 gives alcohols. The addition of borane to the double bond takes place in such a manner that boron atom gets attached to that carbon atom of the double bond which has greater number of hydrogen atoms. This two step process is called hydroboration – oxidation and gives alcohol which seem to have been formed by anti – Marovnikov ‘s addition of water to alkenes.
3) From Carbonyl Compounds
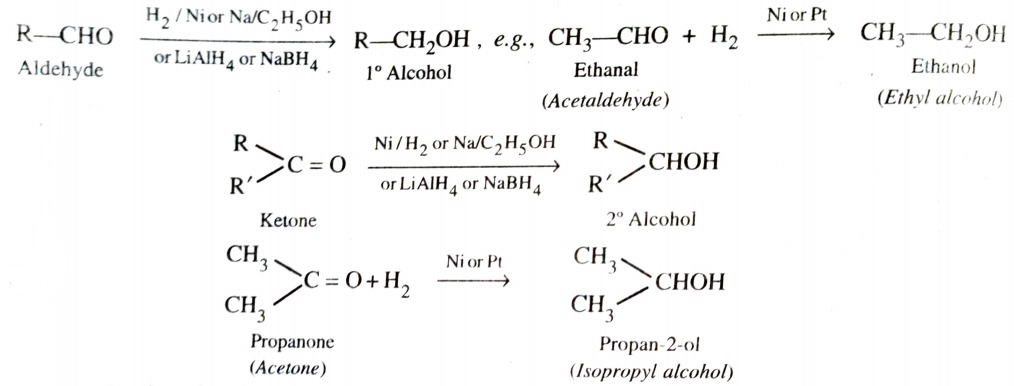
a) By Reduction of Aldehydes and Ketones
Aldehydes and Ketones are reduced to the corresponding alcohols i)by addition of H2 in presence of a catalyst (catalytic hydrogenation) , such as finely divided platinum , palladium , nickel or ruthenium , ii) by action of sodium on ethanol and iii) by complex metal hydrides such as lithium aluminium hydride (LiAlH4) or sodium borohydride (NaBH4) .
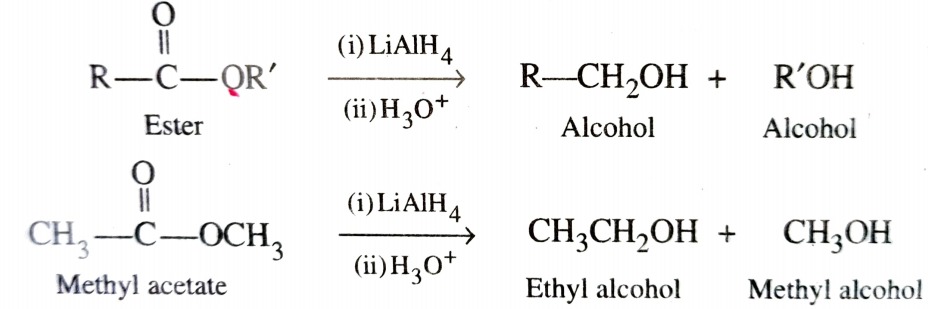
b) By Reduction of Carboxylic Acids and Esters
Reduction of carboxylic acids with LiAlH4 or better with B2H6 gives primary alcohol.
Direct reduction of esters to alcohols can be achieved either with LiAlH4 or Na/alcohol . Unlike carboxylic acid reduction of esters gives a mixture of two alcohols – one from acyl group and other from alkoxy group .
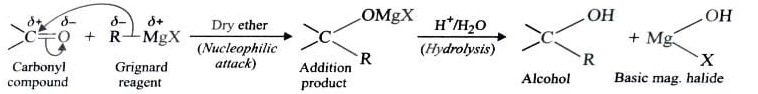
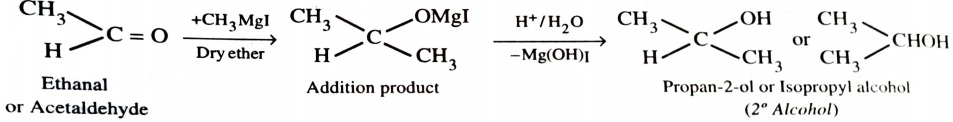
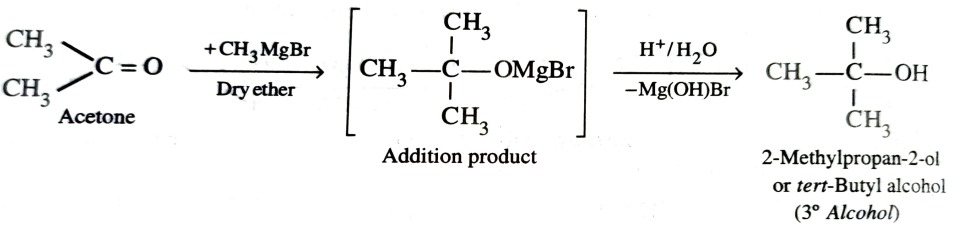
4) From Grignard Reagents
As C—Mg bond is polar , grignard reagent serve as potential source of carbanions . They react with aldehyde , ketones and esters to form alcohols in two steps . The first step involves the nucleophilic attack of the grignard reagent to the carbonyl group to form an adduct (addition product) . The second step involves hydrolysis of the adduct with water or preferably with dil. HCl or dil. H2SO4 to form an alcohol .
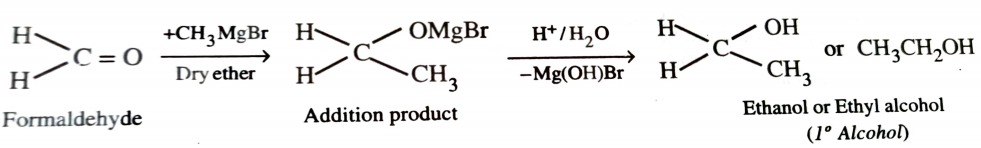
When formaldehyde reacts with grignard reagent , primary alcohols are formed.
With aldehydes other than formaldehyde , secondary alcohols are obtained.
With ketones , tertiary alcohols are obtained.
5) From Aliphatic Primary Amines
Aliphatic primary amines on treatment with nitrous acid gives primary alcohols.
R- CH2-NH2 (1 amine) + HONO (Nitrous acid) → R-CH2-OH (1 alcohol) + N2 + H2O
CH3-CH2-NH2 (Ethylamine) + HONO (Nitrous acid) → CH3-CH2-OH (Ethyl alcohol) + N2 + H2O
C6H5-CH2-NH2 (Benzylamine) + HONO (Nitrous acid) → C6H5-CH2-OH (Benzyl alcohol) + N2 + H2O
Preparation of Phenols
Phenols are also known as carbolic acid.
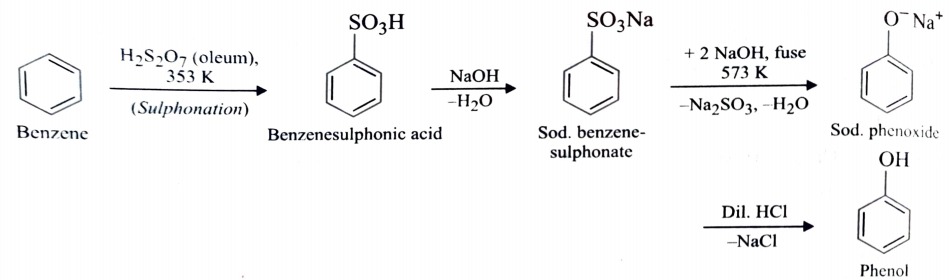
1) From Benzenesulphonic Acid
Benzene is sulphonated with oleum and benzenesulphonic acid thus formed is fused with NaOH at 623K when sodium phenoxide is formed . Acidification of sodium phenoxide gives phenol.
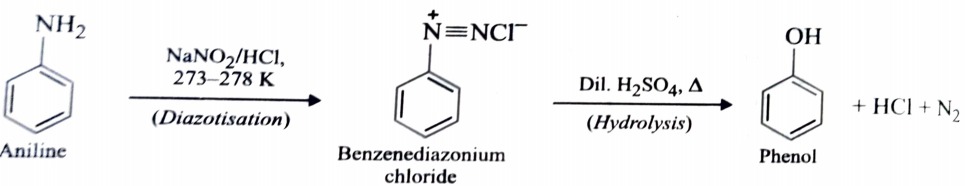
2) From Diazonium Salts
This is one of the most convenient method for preparation of phenols. A diazonium salt is formed by treating an aromatic primary amine with nitrous acid at 273- 278K . When diazonium salt solution are warmed with water or dilute acids , they undergo hydrolysis to form phenols.
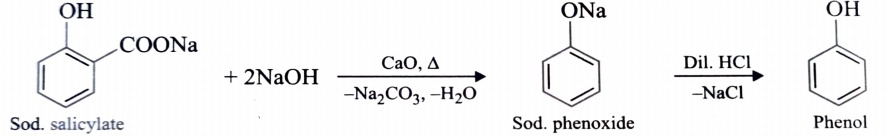
3) From Decarboxylation of Salicylic Acid
Phenol is also obtained by decarboxylation of sodium salt of salicylic acid with soda- lime followed by acidification with dil. HCl
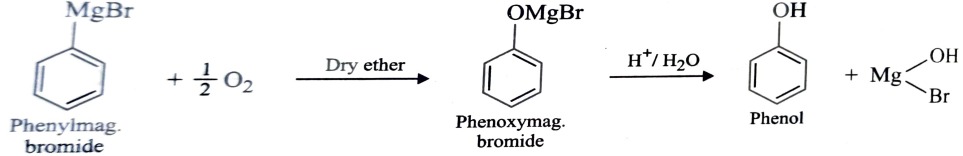
4) From Grignard Reagent
When oxygen is bubbled through an ethereal solution of phenylmagnesium bromide , it forms an adduct product which upon treatment with dil. mineral acid gives phenol.
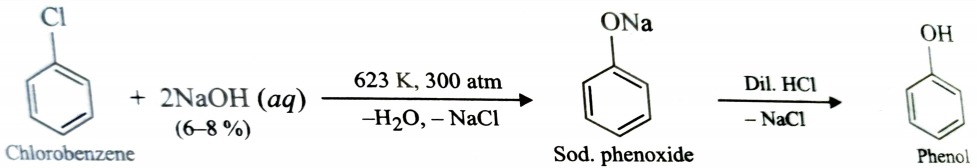
5) From Aryl Halides (Dow’s Process)
Chlorobenzene when treated with aqueous solution of NaOH at 623 K under 300atm pressure, gives sodium phenoxide which upon acidification gives phenol . This reaction is called Dow’s process .
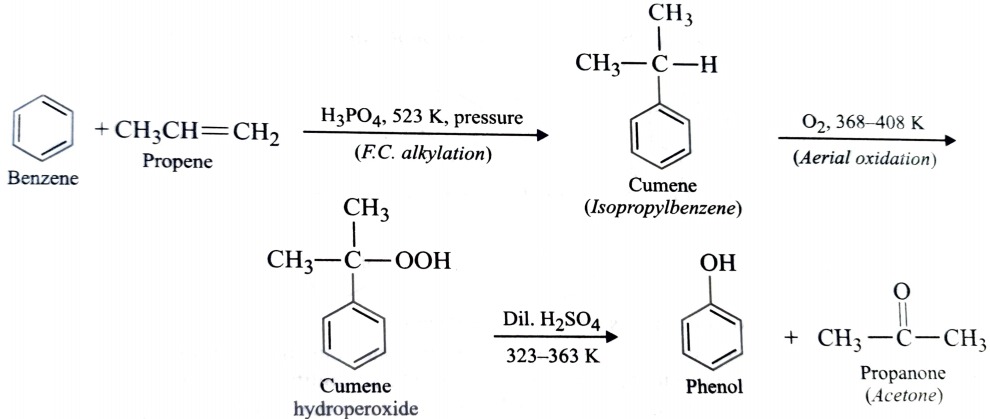
6) From Cumene
Phenol is prepared commercially from cumene which in turn is prepared by Friedel Crafts alkylation of benzene with propene in presence of phosphoric acid. This on aerial oxidation forms cumene hydroperoxide which upon subsequent hydrolysis with an aqueous acid gives phenol and propanone.
Physical Properties of Alcohols and Phenols
Physical State
- At ordinary temperature , lower alcohols are colourless liquids with distinct smell and burning taste . The higher members are colourless , odourless waxy solids.
- Likewise pure phenols are either colourless liquids or solids . But they usually turn reddish brown due to atmospheric oxidation .
Boiling Points
- The boiling point of alcohols are higher that corresponding aliphatic hydrocarbon and haloalkane due to presence of intermolecular hydrogen bonding .
- The boiling point of isomeric alcohols decrease with branching due to corresponding decrease in surface area ,i.e., boiling point decrease in the order : Primary > Secondary > Tertiary
- With increase in molecular mass , boiling point show a regular increase.
- Boiling point of phenols are higher than corresponding aromatic hydrocarbons and haloarenes sice phenols also form intermolecular hydrogen bonds.
Solubility
- The lower alcohols are soluble in water but solubility decrease with increase in molecular mass of the alcohol.
- The extent of solubility of an alcohol in water depends upon the capability of its molecules to form hydrogen bonds with water. As the molecular mass increases , the hydrocarbon part becomes large which resists the formation of hydrogen bonds with water molecules and hence the solubility goes on decreasing .
- Among isomeric alcohols , solubility increases with branching . This is due to the reason that as branching increases , the surface area of non polar hydrocarbon part decreases and hence solubility increases.
- Phenols also form hydrogen bonds with water and hence soluble in water. The solubility of phenols in water is much lower than that of alcohols because of large hydrocarbon part present in their molecules.
Chemical Reactions of Alcohols and Phenols
a)Reactions Involving Cleavage of O—H Bond
1.Acidity of Alcohols and Phenols
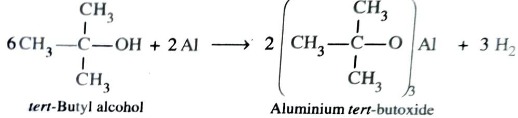
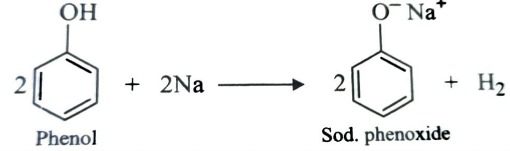
Reaction with Metals
Both alcohols and phenols are acidic in nature and hence react with active metal to form metal alkoxides and phenoxides respectively with evolution of hydrogen gas.
2ROH (Alcohol) + 2M → 2R—OM + H2
2CH3-OH (Methanol) + 2Na →2 CH3-ONa (Sodium methoxide) + H2
2CH3-CH2-OH (Ethanol) + Mg →( CH3-CH2-O)2Mg (Magnesium ethoxide ) + H2
Acidity of Alcohols
Alcohols are weak acids even weaker than water . In water O is attached to two H atoms but in alcohol O is attached to one H atom and one alkyl group . R group has +I effect which increases the electron density in O–H bond in alcohol as compared to that in O–H bond in water . In other words , O–H bond in alcohol is stronger than O–H bond in water. Thus , abstraction of proton from alcohol by a strong base is little more difficult than water. Hence , alcohols are weaker acids than water due to +I effect .
O–H bond in tertiary alcohol is strongest due to max number of alkyl group . thus acidic strength of alcohol follows the order : primary > secondary > tertiary
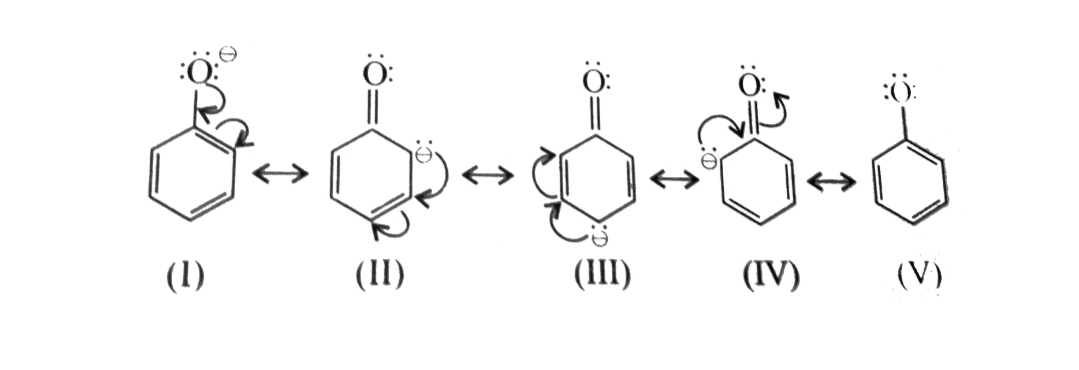
Acidity of Phenols
Phenols are stronger acid than alcohols because phenoxide ion left after the release of proton is stabilized by resonance but the alkoxide ion is not . In alkoxide ion , the negative charge is localized on oxygen while in phenoxide ion , the charge is delocalised.
Effect of EWG on Acidity of Phenols
Electron withdrawing group like -NO2 , -CN , -X etc which stabilize the phenoxide ion by dispering the negative chaerge relative to phenol increase the acidic strength of phenols. The effect is more pronounced at o- and p- position than at m-positions wrt the —OH group .
Greater the number of electron withdrawing group , more acidic is phenol
2,4,6-Trinitrophenol > 2,4-Dinitrophenol > 4-Nitrophenol > 2-Nitrophenol > 3-Nitrophenol > Phenol
Effect of EDG on Acidity of Phenols
Electron donating group like -NH2 , -OH , -OR donates the electron , intensifies the negative charge , destabilizes the phenoxide ion w.r.t phenol and thus decreases the acidic strength .
Acid strength of cresols w.r.t phenol decreases in the order : Phenol > m-Cresol > p-Cresol > o-Cresol
2. Esterification
Alcohols react with carboxylic acids , in presence of few drops of conc . H2SO4 or dry HCl gas as catalyst .This reaction which is slow and reversible is called esterification .
RCOOH (carboxylic acid ) + H—OR’ ⇔ RCO—OR’ (Ester) + H2O
CH3COOH (Ethanoic acid) + HOCH2CH3 (Ethanol) ⇔ CH3CO—OCH2CH3 (Ethyl ethanoate) + H2O
In contrast , phenols cannot be converted into esters by direct reaction with carboxylic acids , the reason being that phenols are less nucleophilic than alcohols due to resonance stabilization and hence fail to attack the carbonyl group of carboxylic acids to form esters.
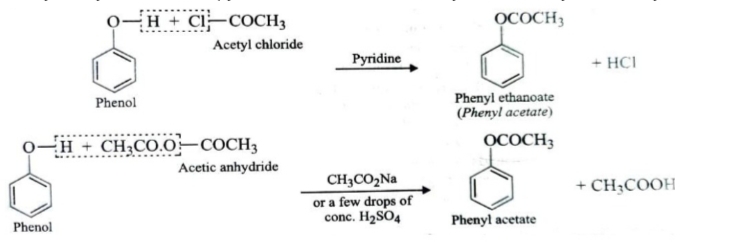
3.Reaction with Acid Chlorides and Anhydrides
When alcohols or phenols are treated with acid chlorides or anhydrides , the H atom of OH group is replaced by the acyl group to form esters.
RCOCl (Acid chloride ) + H—OR’ (Alcohol) → RCOOR’ (Ester) + HCl
(RCO)2O (Acid anhydride ) + H—OR’ (Alcohol) → RCOOR’ (Ester) + RCOOH (Acid)
Phenols also react with acid chlorides and anhydrides to give esters in excellent yields. The reaction with acetyl chloride is usually catalysed by base such as pyridine , that with acetic anhydride is catalysed both by acids and bases.
b) Reaction Involving Cleavage of Carbon-Oxygen (C—O) Bond in Alcohol
1)Reaction with Phosphorous Halides
Phosphorous halide react with alcohol to form haloalkane or alkyl halide in excellent yields (80% or above).
ROH (Alcohol) + PCl5 (Phosphorous pentachloride) → R—Cl (Chloroalkane) + POCl3 (Phosphorous oxychloride) + HCl
CH3CH2OH (Ethanol) + PCl5 (Phosphorous pentachloride) → CH3CH2-Cl (Chloroalkane) + POCl3 + HCl
3ROH(Alcohol) + PCl3 (Phsphorous trichloride) → 3R-Cl (Alkyl chloride ) + H3PO3 (phosphoric acid)
In contrast , treatment of phenol with PCl5 gives only a small amount of chlorobenzene.
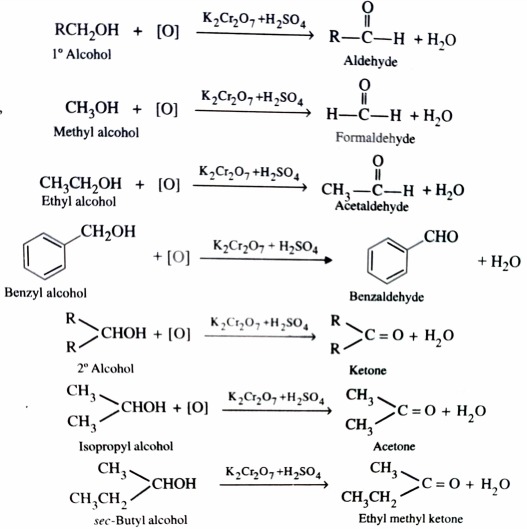
2)Oxidation
Alcohols on controlled oxidation give aldehyde or ketone. Whereas Primary alcohol give aldehydes , secondary alcohols give ketones . The common oxidising agent used are acidified K2Cr2O7 , aqueous or alkaline KMnO4 and chromic anhydride.
The aldehyde formed in this process are readily oxidised to give carboxylic acids if allowed to remain in the reaction mixture .
Therefore , to prevent further oxidation of aldehydes to carboxylic acids , the aldehydes are distilled off as soon as they are formed .
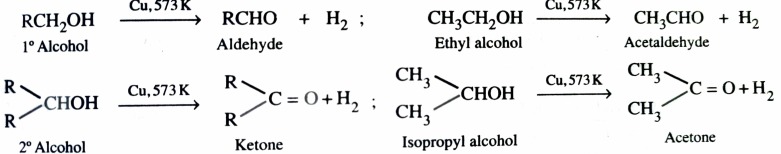
3)Catalytic Dehydrogenation of Alcohols
The dehydrogenation of alcohols is carried out by passing the vapours of alcohol over reduced copper at 573K . Primary alcohols give aldehyde while secondary alcohol give ketones .
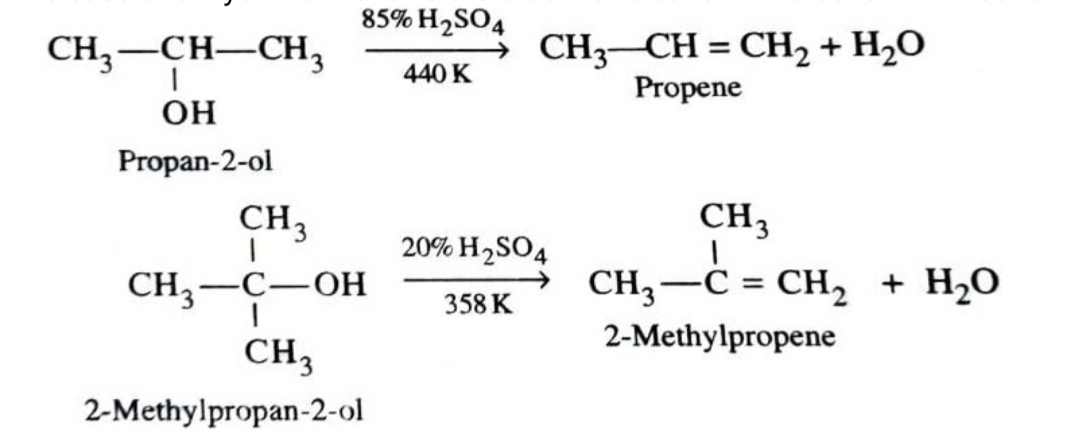
4)Dehydration
Dehydration (removal of a molecule of water) of alcohols can lead to formation of either alkenes or ethers. This dehydration can be carried out either with protonic acids such as conc. H2SO4 , H3PO4 or catalysts such as zinc chloride or alumina .
The ease of dehydration follows the order : 3º alcohol > 2º alcohol > 1º alcohol
5)Reaction with Hydrogen Halides
For a given alcohol , the reactivity of halogen acids decreases in order : HI > HBr > HCl and that of alcohols for a given halogen acid follows the order : tertiary > secondary > primary
R—OH (Alcohol) + HX → R—X (Haloalkane) + H2O
The above reaction is not applicable for preparation of aryl halide because carbon- oxygen bond in phenol has a partial double bond character and is difficult to break being stronger than a single bond.
c) Reactions of Phenols
1)Electrophilic Aromatic Substitution
Phenols undergo electrophilic substitution readily and it is usually difficult to prevent polysubstitution . Further , since the OH group increases electron density more at o- and p-positions than at m-positions , therefore , OH group is ortho , para – directing .
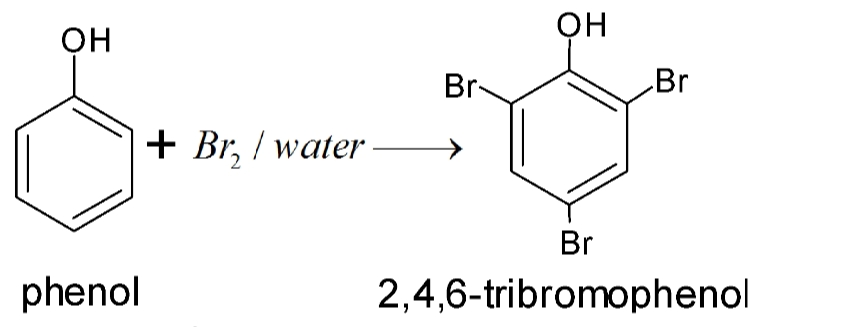
Halogenation
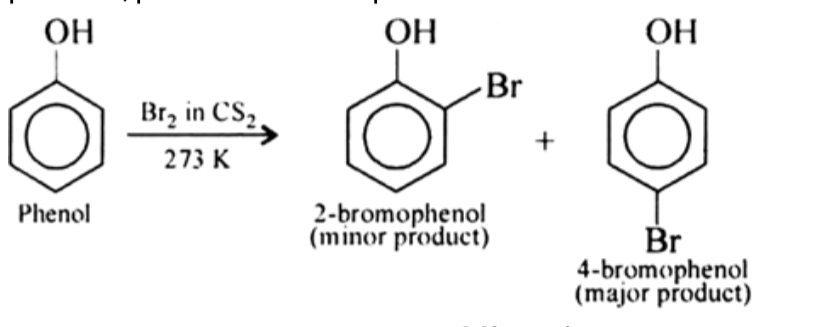
Phenols when treated with chlorine or bromine water gives polyhalogen derivatives in which all the H atom present at the ortho and para positions with respect to OH group are replaced by chlorine or bromine atoms. In aqueous solution , phenol ionizes to form phenoxide ion . Due to presence of negative charge , the oxygen of phenoxide ion donates electron to the benzene ring to a large extent . Thus , ring gets highly activated and hence tri-substitution occurs.
In presence of less polar solvent such as CS2 , CHCl3 , CCl4 etc and at low temperature , monophenols are obtained. In non polar solvent , ionization of phenol is greatly suppressed . Thus oxygen of OH group donates electron to benzene ring only to a small extent. Thus , ring is activated slightly and hence mono-substitution occurs . Further , because of steric hindrance at ortho position , para substitution predominates .
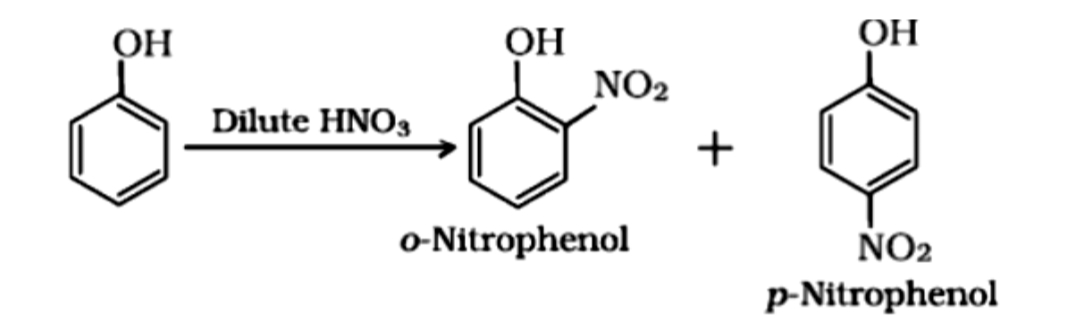
Nitration
With dilute nitric acid at 293 K , phenol gives mononitrophenols, viz., a mixture of 2- and 4- nitrophenols. However , here 2-nitrophenol predominates over 4-nitrophenol due to stabilization of transition state by hydrogen bonding .
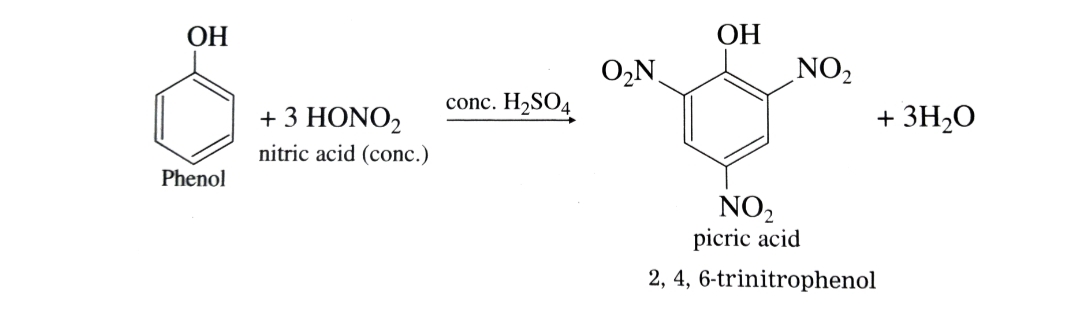
With concentrated nitric acid in presence of concentrated H2SO4 phenol gives 2,4,6-trinitrophenol (picric acid) . The yield is poor since most of the phenol is oxidised by concentrated nitric acid.
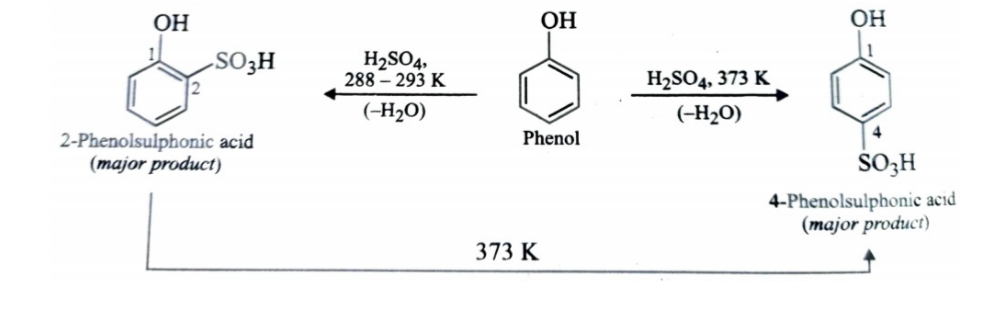
Sulphonation
Sulphonation of phenol gives a mixture of ortho and para-isomers . If the sulphonation is carried out at low temperature, kinetically controlled ortho isomer predominates and if sulphonation is carried out at high temperature , thermodynamically controlled para isomer is the chief product.
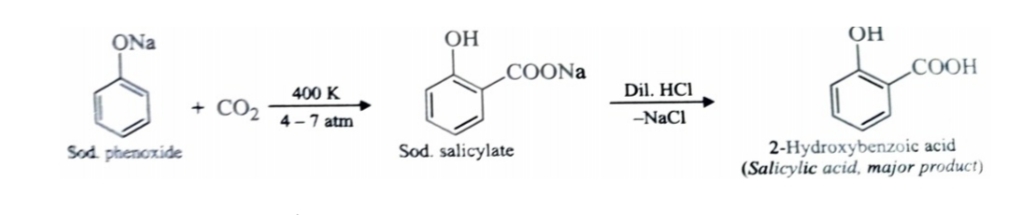
2)Kolbe’s Reaction
Sodium phenoxide reacts with carbon dioxide under pressure (4-7) atmosphere at 400K to form sodium salicylate which upon acidification with mineral acids gives salicylic acid . This reaction is called kolbe reaction .
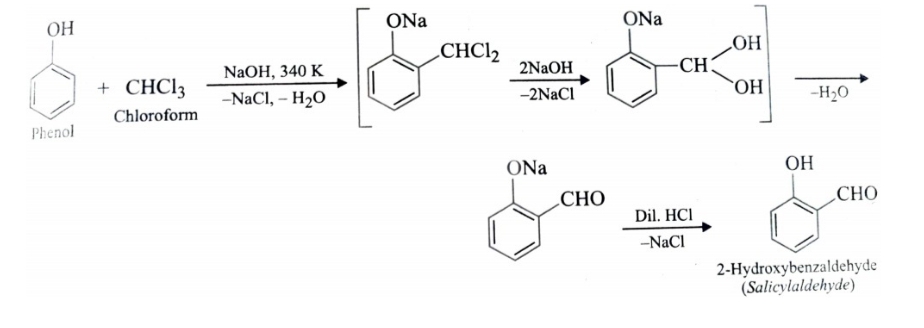
3)Riemer – Tiemann Reaction
Treatment of phenol with chloroform in presence of aqueous sodium or potassium hydroxide at 340 K followed by hydrolysis of resulting product gives 2-hydroxybenzaldehyde (salicylaldehyde) . This reaction is called Riemer Tiemann reaction .
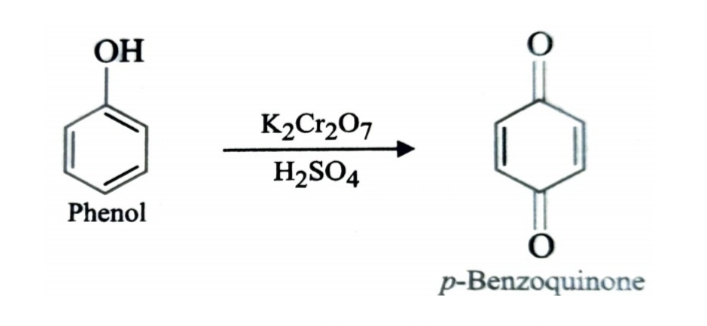
4)Oxidation of Phenol
Oxidation of phenol with chromic acid produces a diketone known as p-benzoquinone. In presence of air , phenols are slowly oxidised to dark coloured mixtures containing quinones.
Preparation of Ethers
1)Williamson Synthesis
Haloalkanes when treated with sodium or potassium alkoxides form ethers or alkoxyalkanes.
R—X + NaOR’ → R—O—R’ + NaX
CH3CH2-Br (Bromoethane) +NaOCH2CH3 → CH3CH2 —O—CH2CH3 (Ethoxyethane) + NaBr
CH3I + NaOCH3 → CH3 —O—CH3 + NaI
This reaction is called Williamson synthesis.
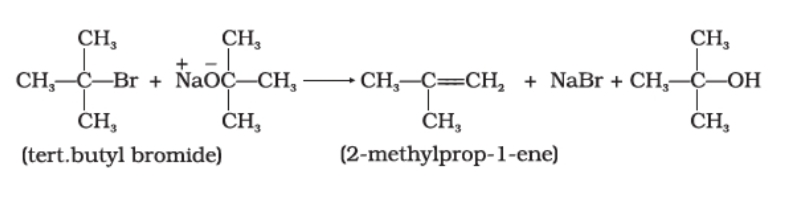
With secondary alkyl halides both substitution and elimination occur giving a mixture of ethers and alkenes but with tertiary alkyl halide only elimination occurs giving alkenes .
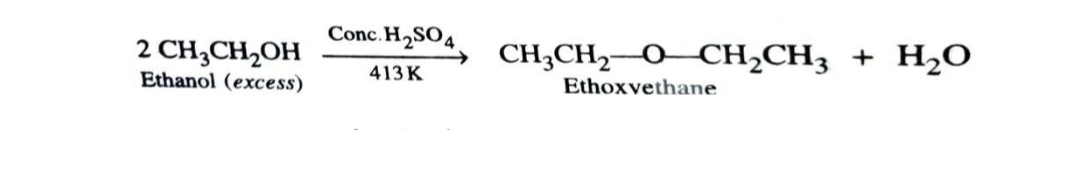
2)Dehydration of Alcohols
Alcohols (excess) undergo dehydration in presence of protic acids to give ethers. The formation of ether is a bimolecular nucleophilic substitution reaction .
This method is not preferred with secondary and tertiary alcohol since reaction occurs by SN1 mechanism and alkene is the major product .
3)By Reaction of Alkyl Halide with Dry Silver Oxide
Ethers can also be prepared by heating an alkyl halide with dry silver oxide.
Physical Properties of Ethers
Physical State , Colour and Odour
Dimethyl ether and ethyl methyl ether are gases at ordinary temperature while the other lower homologues of ether are colourless liquid with ether smell.
Boiling Points
Ethers are isomeric with monohydric alcohol but their boiling point are much lower than those of isomeric alcohol because ether do not form hydrogen bonds. As a result , ethers do not show molecular association and hence have lower boiling point than corresponding alcohols.
Solubility
The solubility of lower ethers in water is due to the formation of hydrogen bonds between water and ether molecules.
As the molecular mass increases , the solubility of ether in water decreases due to corresponding increase in hydrocarbon portion of the molecule .
Chemical Properties of Ethers
1)Reaction Involving Cleavage of Carbon Oxygen Bond
With Halogen Acids
Ethers are readily cleaved by hydroiodic acid or hydrobromic acid at 373K to form an alcohol and an alkyl halide.
For Symmetrical Ethers : R—O—R (Ether) + HX → R—OH (Alcohol) + R—X (Alkyl halide )
For unsymmetrical ethers , there are different cases as discussed below:
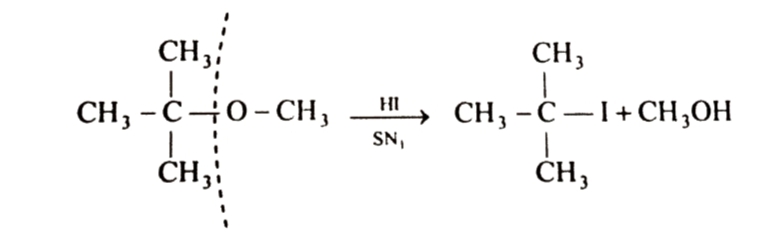
If one of the alkyl group is tertiary , alkyl halide is formed from tertiary alkyl group because reaction now occurs by SN1 mechanism and formation of product is controlled by stability of carbocation . Since a tert-butyl carbocation is more stable than methyl carbocation , therefore cleavage of C—O bond gives methyl alcohol and the more stable tert-butyl carbocation which reacts with I- ion to form tert butyl iodide.
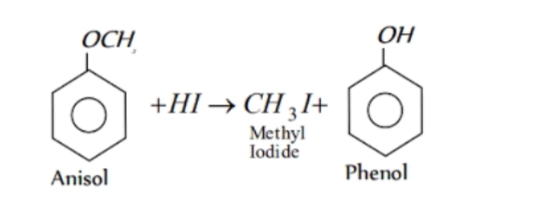
In case of an alkyl aryl ether , products are always phenol and an alkyl halide and never an aryl halide and an alcohol .
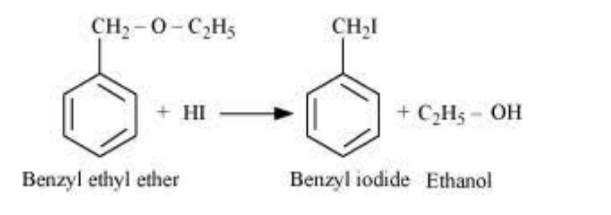
In case of benzyl alkyl ethers such as benzyl ethyl ether ,the reaction proceeds by SN1 mechanism . Since benzyl carbocation is more stable than methyl carbocation , therefore cleavage of C—O bond gives ethyl alcohol and benzyl carbocation . This carbocation then reacts with I- ion to form benzyl iodide.
Electrophilic Substitution Reaction
In aryl alkyl ethers , the +R effect of the alkoxy group increases the electron density in the benzene ring thereby activating the benzene ring towards electrophilic substitution reaction .
Since electron density increases more at ortho and para position as compared to meta position , therefore , electrophilic substitution reactions mainly occur at o- and p-positions.
# Alcohols Phenols and Ethers
# Alcohols Phenols and Ethers Notes
# Alcohols Phenols and Ethers Solutions
# Alcohols Phenols and Ethers NCERT Solutions
# Alcohols Phenols and Ethers Notes in Detail
Do share this post if you liked Alcohols Phenols and Ethers Class 12. For more updates, keep logging on BrainyLads