Amines Class 12 | Chapter 13 | Chemistry | Notes | CBSE |
Table of Contents
Amines Class 12 | Chapter 13 | Chemistry | Notes | CBSE |
Amines
Amines Class 12 : Amines are the derivatives of Ammonia, obtained by replacement of one, two or all the three hydrogen atoms by alkyl or aryl groups.
Structure of Amines
- Like Ammonia , Nitrogen atom of amines is trivalent .
- Nitrogen atom in amines is sp3 hybridized.
- Each of three sp3 hybridized orbital overlap with orbital of hydrogen or carbon.
- In all amines , fourth sp3 orbital contains the lone pair of electrons.
- Since lone pair – bond pair repulsion are much more than bond pair – bond pair repulsion , the bond angle between two adjacent H atom or alkyl group decreases from 109.28′ to 107º in 1º and 2º amine while due to steric hinderance it is 108º in 3º amines .
Classification of Amines
NH3 (Ammonia) NH2—R (Primary Amine )
NH2—R R—NH—R’ (Secondary Amine )
R—NH—R’ 
Amines are further classified into two categories :-
1) Aliphatic Amines : Amines in which the nitrogen atom is directly linked to one , two or three (same or different ) alkyl groups are called aliphatic amines .
2) Aromatic Amines : Amines in which the nitrogen atom is directly linked to one , two or three (same or different ) aromatic rings or aryl groups are called aromatic amines .
Nomenclature of Aliphatic Amines
| Amines | Common Name | IUPAC Name |
| Primary Amines | Alkylamine or Aminoalkane | Alkanamine |
| CH3NH2 | Methylamine or Aminomethane | Methanamine |
| CH3CH2NH2 | Ethylamine or Aminoethane | Ethanamine |
| CH3CH2CH2NH2 | n-Propylamine or 1-aminopropane | 1-Propanamine or Propan-1-amine |
 |
Isopropylamine or 2-Aminopropane | 2-Propanamine or Propan-2-amine |
| Secondary Amine | Alkylamine or
N-Alkylaminoalkane |
N-Alkylalkanamine |
| CH3-NH-CH3 | Dimethylamine or
N-methylaminomethane |
N-Methylmethanamine
|
| CH3-CH2-NH-CH3 | Ethylmethylamine or
N-Methylaminomethane |
N-Methylethanamine |
| Tertiary Amine | Alkylamine or
N-Alkyl-N-alkylaminoalkane |
N-Alkyl-N-alkylalkanamine |
 |
Trimethylamine or
N,N-Dimethylaminomethane |
N,N-Dimethylmethanamine |
 |
Diethylmethylamine or
N-Ethyl-N-methylaminoethane |
N-Ethyl-N-methylethanamine |
Nomenclature of Aromatic Amines
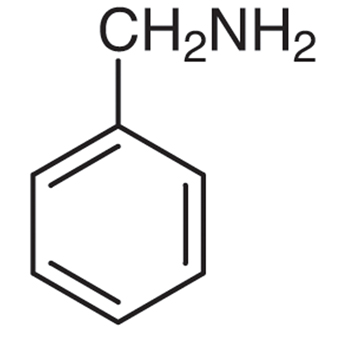

Common: Aniline Benzylamine N-Methylaniline N,N-Dimethylaniline
IUPAC: Benzenamine 1-Phenylmethanamine N-Methylbenzenamine N,N-Dimethylbenzenamine



Common: o-Toluidine m-Toluidine o-Anisidine N-Phenylaniline
IUPAC : 2-Methylbenzenamine 3-Methylbenzenamine 2-Methoxybenzenamine N-Phenylbenzenamine
Preparation of Amines
1) Reduction of nitro compounds
Both aliphatic and aromatic primary amine can be easily prepared by reduction of the corresponding nitro compounds.
Reagent :
- Hydrogen in presence of Raney Ni
- Finely divided Pt or Pd
- Active metal such as Fe , Sn , Zn in presence of concentrated hydrochloric acid.
- CH3CH2—NO2 (Nitroethane) + 3H2
CH3CH2– NH2 (Ethylamine) + 2H2O
- CH3CH2—NO2 + 6[H]
CH3CH2– NH2 (Ethylamine) + 2H2O
- C6H5-NO2 + 3H2
C6H5-NH2 + 2H2O
Reduction with iron scrap and hydrochloric acid is preferred over Sn/HCl because FeCl2 formed gets hydrolysed to release hydrochloric acid during the reaction . Thus ,only a small amount of hydrochloric acid is required to initiate the reaction .
2) Ammon lysis of Alkyl Halides
When an alkyl or benzyl halide is heated with an alcoholic solution of ammonia in a sealed tube at 373K , a mixture of 1º , 2º and 3º amines with some quaternary ammonium salt is obtained.
Ammonolysis has a disadvantage of yielding a mixture of primary , secondary , tertiary amines and quaternary ammonium salt .
Since , aryl halides are much less reactive than alkyl halides towards nucleophilic substitution reactions , therefore , aniline and other arylamines cannot be prepared by this method .
3) Reduction of Nitriles
Aliphatic and aralkyl primary amines can be easily prepared by the reduction of corresponding nitriles either catalytically with H2 and Raney Nickel or chemically with lithium aluminium hydride or sodium and alcohol or sodium amalgam and alcohol .
Reduction with Sodium and alcohol is called Mendius reduction .
- R—C≡N (alkyl nitrile) or Ar—C≡N (Aryl nitrile )
RCH2NH2 or ArCH2NH2
- CH3C≡N (Acetonitrile )
CH3CH2NH2 (Ethylamine)
This reaction gives us an excellent method for converting alkyl halides into primary amines having one carbon atom more than the parent alkyl halide.
4) Reduction of Amides
Primary , secondary and tertiary amines can be prepared by the reduction of the corresponding amides with lithium aluminium hydride .
R—CONH2 (1º amide ) R–CH2NH2 ( 1º amine )
CH3—CONH2 (Acetamide) CH3CH2NH2 (Ethylamine)
C6H5—CONH2 ( Benzamide) C6H5 CH2NH2 (Benzylamine )
CH3CONHCH3 (N-Methylacetamide) CH3CH2NHCH3 (Ethylmethylamine) (2º)
5) Gabriel Phthalimide Synthesis
In this reaction , phthalimide is converted into potassium salt by treating it with alcoholic potassium hydroxide . Then potassium phthalimide is heated with an alkyl halide to yield an N-alkylphthalimide which is hydrolysed to phthalic acid and a primary amine by heating with HCl or KOH solution .
Aromatic primary amine such as aniline cannot be prepared by this method since aryl halides do not undergo nucleophilic substitution under normal conditions .
6) Hoffmann Bromamide Degradation Reaction
The conversion of primary amide to a primary amine containing one carbon atom less than the original amide on heating with a mixture of Br2 in presence of NaOH or KOH is called Hoffmann Bromamide reaction or Hoffmann degradation of amides .
CH3CONH2 (Acetamide) + Br2 + 4NaOH → CH3NH2 (Methanamine) + 2NaBr + Na2CO3 + 2H2O
CH3CH2CONH2 (Propionamide) +Br2 +4NaOH →CH3CH2NH2(Ethylamine) + 2KBr +K2CO3 +2H2O
C6H5CONH2 (Benzamide) + Br2 + 4NaOH → C6H5CH2NH2 (Aniline)+ 2KBr + K2CO3 + 2H2O
7) Reduction of Oximes
Primary amines can be prepared by the reduction of oximes of aldehyde and ketones with either lithium aluminium hydride / ether or with sodium and alcohol .
RCH=NOH (Aldoxime ) R–CH2NH2


Physical Properties of Amines
- Pure amines are almost colourless but develop colour on keeping in air for long time . This is due to the reason that amines especially aromatic amines are readily oxidised in air to form coloured oxidation product.
- Amines have higher boiling point than hydrocarbon of comparable molecular masses . This is due to the reason that amines being polar form intermolecular hydrogen bonding ( except tertiary amines which do not have hydrogen atoms linked to nitrogen atom ) .
- The degree of association depends upon the extent of H-Bonding . 1º amine has the highest while 3º amines have the lowest boiling point .
- As the size of alkyl group increases (with increase in molecular mass ) , the solubility decrease due to corresponding increase in hydrocarbon part (hydrophobic part ) of the molecule .
- Aromatic Amines on the other hand are insoluble in water . This is due to large hydrocarbon part which tends to retard the formation of H-bonds . Thus , aniline is almost insoluble in water . However , it is quite soluble in organic solvents such as benzene , ether , alcohol etc.
Note : Amines behave as nucleophile due to presence of unshared pair of electron.
Chemical Reactions of Amines
1) Basic Character of Amines
Amines are quite reactive organic compounds due to
- Difference in electronegativity of nitrogen and hydrogen atom
- the presence of lone pair of electron on Nitrogen atom
All types of Amines react with mineral acids such as HCl , HNO3 , H2SO4 , etc .
Note : Amine salt on treatment with a base like NaOH regenerate the parent amine.
RNH3+X– + OH– → RNH2 : + X– + H2O
Note : Amine salts are soluble in water but insoluble in organic solvent like ether.
The basic strength of an amine is determined by its basicity constant , Kb
Greater the value of basicity constant , stronger is the acid Or smaller the value of pKb , stronger is the acid.
Basicity of Aliphatic Amines
Aliphatic amines are stronger bases than ammonia :-
All the three classes of aliphatic amines are stronger bases than ammonia . This is due to the reason that alkyl groups are electron donating group . This is due to the reason that alkyl groups are electron donating groups . As a result , the electron density on the nitrogen atom increases and thus they can donate the lone pair of electron more easily than ammonia .
Thus , the order of basicity of amines in gaseous phase is as follows :
3º amine > 2º amine > 1º amine > Ammonia
However , it has been found that in aqueous solution , 2º amine is more basic than 1º and 3º amine .
Basicity order :
(CH3)2NH > CH3NH2 > (CH3)3N > NH3 ( 2º amine > 1º amine > 3º amine > Ammonia)
(C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3 ( 2º amine > 3º amine > 1º amine > Ammonia)
The basicity of amine in aqueous solution primarily depends upon the stability of the ammonium cation or the conjugate acid formed by accepting a proton from water . The stability of the ammonium cation, in turn , depends upon combination of following three factors :
- + I – Effect of the alkyl group
- Extent of H-Bonding with water molecule
- Steric effects of the alkyl group
Basicity of Aromatic Amines
Aromatic amines are far less basic than ammonia and aliphatic amines.
i) Due to resonance in aniline : In aromatic amines NH2 group is directly attached to benzene ring due to which lone pair of electron on nitrogen atom of ammonia is delocalized over the benzene ring and is thus less available for protonation .
Aniline is a resonance hybrid of five structures
ii)Lower stability of anilinium ion than aniline : Anilinium ion is resonance hybrid of only two structures . We know lesser is number of resonating structures , lesser is stability . Thus , Aniline is more stable than anilinium ion .
Effect of Electron Donating Group on Basicity of Aromatic Ring
Electron donating group such as –CH3 , –OCH3 , –NH2 releases electrons , stabilizes the conjugate base and thus increases the basic strength .
Relative basic strength of toluidines
- Here , CH3 group show both +I effect and hyperconjugation effect at p position .
- At m position it shows only + I effect and thus less basic than p-toluidine.
Relative basic strength of methoxyanilines ( anisidines )
- Unlike methyl group , OCH3 group shows both electrondonating resonance effect (+R effect ) as well as electron withdrawing inductive effect (-I effect )
- At m position it exert only -I effect which decreases the electron density on Nitrogen atom and thus is least basic.
- p-Anisidine is strongest base due to +R effect of OCH3 group .
Effect of Electron Withdrawing Group on Basicity of Aromatic Ring
Electron withdrawing group such as –NO2 , –CN , –X , –COOH etc withdraws electrons from benzene ring destabilizes the conjugate ion and thus decreases the basic strength .
Relative Basic strength of Nitroanilines
- Nitro group has powerful electron withdrawing resonance effect (-R effect) as well as electron withdrawing inductive effect (-I effect ) .
- Due to ortho effect , o-nitroaniline is the weakest base .
- In p-nitroaniline both -R effect and -I effect decreases the density.
- In m-nitroaniline only -I effect decreases the density.
2) Alkylation
The process of introducing an alkyl group into any molecule is called an alkylation .
When treated with excess of ethyl bromide , ethylamine undergoes alkylation to produce a mixture of diethylamine , triethylamine and tetraethylammonium bromide (quaternary ammonium halide )
CH3CH2NH2 (Ethylamine) + CH3CH2Br → (CH3CH2)2NH (Diethylamine) + HBr
(CH3CH2)2NH (Diethylamine) + CH3CH2Br → (CH3CH2)3N (Triethylamine) + HBr
(CH3CH2)3N (Triethylamine) + CH3CH2Br → (CH3CH2)4N+Br – (Tetraethylammonium bromide )
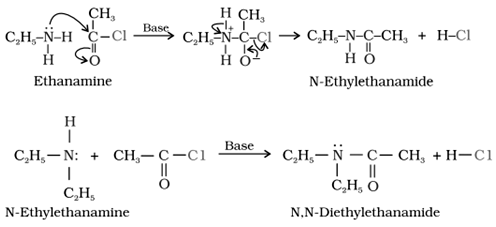
3) Acylation
The process of introducing an acyl group into any molecule is called as acylation .
The product obtained by acylation reaction are known as amides.
It is carried out in presence of base stronger than amine like pyridine which removes HCl formed during the reaction and shifts the equilibrium in forward direction .
4) Benzoylation
The replacement of an active hydrogen of alcohols , phenols or amines by benzoyl group is called benzoylation . It is usually carried out by using benzoyl chloride in presence of aqueous NaOH .
C6H5COCl (Benzoyl chloride ) + HOCH2CH3(Ethyl alcohol)→ C6H5COOC2H3 (Ethylbenzoate) + HCl
C6H5COCl (Benzoyl chloride ) + C6H5OH (Phenol)→ C6H5COOC6H5 (Phenylbenzoate) + HCl
5) Carbylamine Reaction
When a primary amine (aliphatic or aromatic ) is warmed with chloroform and alcoholic KOH , it forms an isocyanide or carbylamines having offensive smell . This reaction is called carbylamine reaction .
R-NH2 + CHCl3 + 3KOH → R-NC + 3KCl + 3H2O
CH3CH2NH2 (Ethylamine) + CHCl3 + 3KOH → CH3CH2 -NC + 3KCl + 3H2O
C6H5NH2 (Aniline ) + CHCl3 + 3KOH → C6H5-NC (Phenyl isocyanide) + 3KCl + 3H2O
Since secondary or tertiary amines (aliphatic or aromatic ) do not give the reaction , it is used as test for primary amines and also for distinction of primary amines from secondary and tertiary amines.
6) Reaction with Nitrous Acid
Primary , secondary and tertiary amines react differently with nitrous acid . Since nitrous acid is unstable , it is prepared insitu by the action of dilute hydrochloric acid on sodium nitrite.
Aliphatic primary amine react with cold nitrous acid to give alcohols with quantitative evolution of Nitrogen gas.
NaNO2 + HCl → HNO2 + NaCl
R-NH2 + HONO → R-OH + N2 + H2O
CH3CH2NH2 (Ethylamine) + HONO → CH3CH2OH + (Ethyl alcohol) + N2 + H2O
Since no other class of ammonia liberates Nitrogen gas on treatment with HNO2 , this reaction is used as a test for aliphatic primary amines .
Aromatic primary amine react with nitrous acid at 273-278K to form arenediazonium salts.
7) Reaction with aryl sulphonyl chloride
Benzenesulphonyl chloride ( C6H5SO2Cl ) is also known as Hinsberg’s reagent .
Reaction of benzenesulphonyl chloride with primary amine is as follows : (Product is soluble in alkali)
A secondary amine gives an insoluble N,N-dialkylbenzenesulphonamide which remin unaffected on addition of acid.
A tertiary amine does not react at all . Therefore , it remains insoluble in the alkaline solution but dissolves on acidification to give a clear solutuion .
Since , all the three types of amines react with benzene sulphonylchloride in different manner . Thus , it is used as test to distinguish between primary , secondary and tertiary amines.
8)Electrophilic Substitution
In addition to the reaction of amino group , aromatic amines also undergo typical electrophilic substitution reactions of the aromatic ring .
In all these reaction , amino group strongly activates the aromatic ring through delocalization of the lone pair of electron of N-atom over the aromatic ring .
As a result , electron density is more at o- and p- positions as compared to m-position. Therefore , the -NH2 group directs the incoming group to o- and p-positions , i.e. , NH2 is an o-, p-directing group .
Bromination
Due to the strong activating effect of the amino group , bromination of amines occurs very fast and bromine enters the p- and both the o- positions even in the absence of a catalyst .
Aniline on treatment with Bromine gives 2,4,6-tribromoaniline
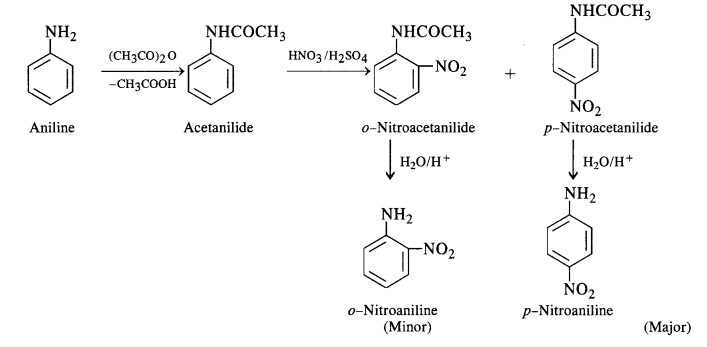
If a monohalogenated derivative is required , the amino group is first acetylated and then halogenation of the ring is carried out . After halogenation , acetyl group is removed by hydrolysis and the monohalogenated amine is obtained . This is called protection of aniline .
By acetylating the amino group , electron pair on Nitrogen atom is transferred towards carbonyl group due to conjugation and thus less available for ring .
p-Bromoaniline is the major product.
Nitration
Nitric acid is not only a nitrating agent but also acts as strong oxidising agent . As a result , direct nitration of aromatic amines is not useful reaction since it often gives tarry oxidation product along with some nitration product .
The reason for formation of large amount of unexpected m-nitroaniline is that under strongly acidic condition of nitration , most of the aniline is converted into anilinium ion and since -NH3+ is a m-directing , thus an unexpected large amount of m-nitroaniline is obtained.
Thus most convenient method for nitration is to first protect the amino group by acetylation .
Sulphonation
In spite of the presence of activating amino group, sulphonation of aromatic amines occurs only under drastic conditions .
Sulphanilic acid contains both an acidic (SO3H) as well as a basic (NH2) group . Thus , sulphanilic acid exists as an internal salt . During the formation of this salt , the amino group has accepted the proton donated by SO3H group . Such type of internal salts are called dipolar ions or zwitter ions . Due to zwitter ion character , it has high melting point and is practically insoluble in water and organic solvents .
Note : Aniline does not undergo friedel craft reaction due to salt formation with aluminium chloride , the Lewis acid which is used as catalyst.
Diazonium Salts
They have the general formula ArN2+Cl–
Primary aliphatic amines form highly unstable alkenediazonium salts . They rapidly decompose even at low temperature whereas Primary aromatic amine form arenediazonium salts , which are stable for short time in solution at low temperature .
The stability of arenediazonium ion is explained on the basis of resonance

Preparation of Diazonium Salts
Aromatic diazonium salts are generally prepared by adding a cold aqueous solution of sodium nitrite to solution of primary aromatic amine in an acid at 273-278 K .
The process of conversion of primary aromatic amine into its diazonium salt is called diazotisation .
Physical Properties of Diazonium Salts
- Arenediazonium salts are generally colourless crystalline solids , highly soluble in water .
- Many diazonium salts especially nitrates are dangerously explosive in the dry state .
- They are never isolated but are usually prepared insitu and used immediately after the preparation .
- Its aqueous solution is good conductor of electricity .
Chemical Properties of Diazonium Salts
A) Reaction Involving Displacement of Nitrogen
- Replacement by Halide or Cyanide ion
Chlorine and Bromine can be easily introduced into the benzene ring by treating a freshly prepared cold aqueous solution of benzenediazonium chloride with a solution of CuCl dissolved in HCl and CuBr dissolved in HBr . This is called Sandmeyer reaction .
Alternatively , Cl ,Br and CN can be introduced into the benzene ring by simply treating diazonium salts with HCl ,HBr and KCN respectively in presence of copper powder instead of Cu salts . This is called Gattermann reaction .
Note : The yield in Sandmeyer reaction is found to be better than Gattermann reaction.
2) Replacement by Iodide Ion
The replacement of diazo group by iodine is simply achieved by treating a diazonium salt with potassium iodide .
3) Replacement by Fluoride Ion
Fluoroarenes cannot be prepared by direct fluorination of aromatic hydrocarbon since the reaction is very violent and cannot be easily controlled. These can be easily prepared by Balz -Schiemann reaction . In this aromatic primary amine is first diazotised with NaNO2 in presence of HBF4 and the aryl diazonium tetrafluoroborate thus formed is heated to give the corresponding aryl fluoride .
4) Replacement by Hydroxyl Group
If the temperature of diazonium salt solution is allowed to rise above 278K , the salt gets hydrolysed to form phenol .
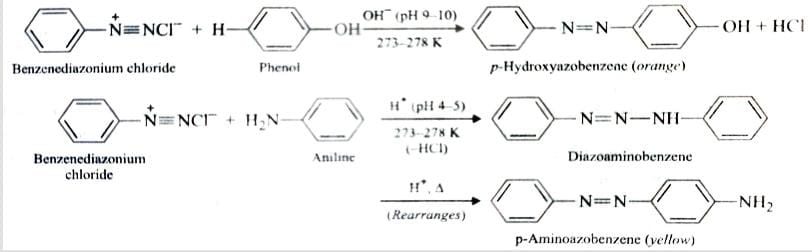
B) Reaction Involving Retention of Diazo Group
Arenediazonium salts react with highly reactive aromatic compounds such as phenol and amines to form brightly coloured azo* compounds. This reaction is known as Coupling reaction .
Coupling with phenols occurs in basic medium while that of amines occurs in faintly acidic medium at 273-278 K . It is an example of Electrophilic Substitution reaction .
# Amines Class 12
# Amines Class 12 Notes
# Amines Class 12 Solutions
# Amines Class 12 NCERT Solutions
# Amines Class 12 Notes in Detail
# Amines Class 12 Types
# Amines Class 12 Examples
Do share this post if you liked Amines Class 12. For more updates, keep logging on BrainyLads