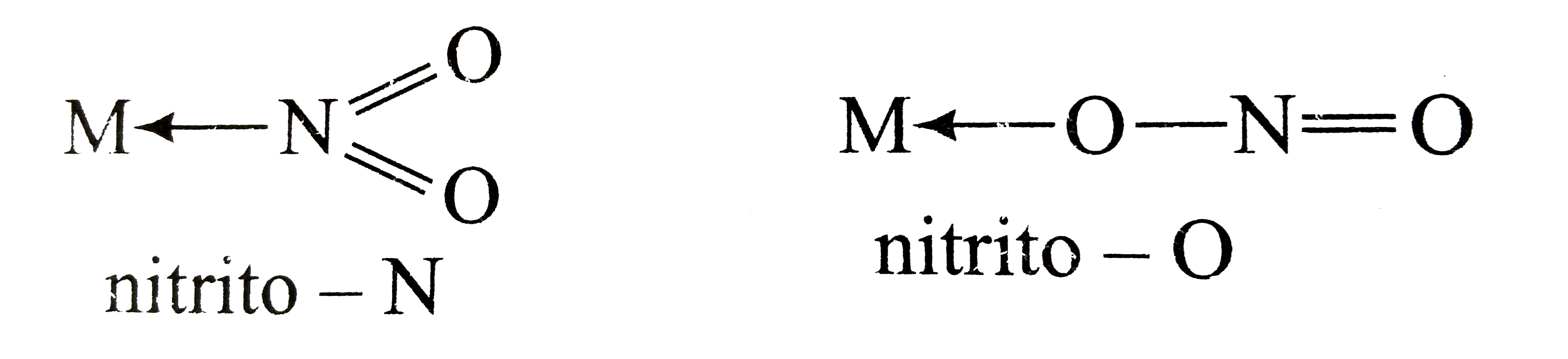Coordination Compounds Class 12 Notes | Chapter 9 | Chemistry |
Table of Contents
Coordination Compounds Class 12 Notes | Chapter 9 | Chemistry | | CBSE |
Coordination Compounds Class 12 Notes
Addition or Molecular Compounds : When solution containing two or more simple stable compound in molecular proportion are allowed to evaporate , crystals of new substance obtained are called addition compounds. It is of two types : Double salts and coordination compounds
Double Salts are the addition compounds which are completely broken down in individual ions when dissolved in water.
Coordination compounds are the compounds in which the central metal atom is linked to a number of ions or neutral molecules by coordinate bonds, i.e., by donation of lone pairs of electrons by these ions or neutral molecules to the central metal atom.
For Example : Nickel tetracarbonyl, [Ni(CO)4] in which CO molecules are linked to the central nickel atom by coordinate bonds by donating lone pair of electrons.
Important Terms Used In Coordination Compounds
Ligands and Central Metal Atom / Ion
The donor atom , molecules or anions which donate a pair of electron to the metal atom or ion and form the coordinate bond with it are called ligands. The metal atom or ion to which these ligands are attached is called central metal atom or ion.
The ligands may be simple ions such as Cl– , small molecules such as H2O , NH3 , large molecules such as ethane-1,2-diamine or even macromolecules such as proteins.
Ligands are Lewis bases while central metal atom or ion is Lewis acids.
Types of Ligands on the Basis of Charges
Anionic Ligands : The ligands which carry negative charge . Example : CN– (Cyanide ion) , H– (Hydride ion) , OH– (hydroxide ion ) , ONO– (Nitrite ion) , NO3– (Nitrate ion), SCN– (Thiocyanate ion), NCS– (Isothiocyanate ion)
Neutral Ligands : The ligands which have lone pair of electrons . Example : NH3 (Ammonia) , H2O (water ) , NO (Nitric oxide ) , PH3 (Phosphine) , CS (Thiocarbonyl), CO (carbon monoxide)
Cationic Ligands : The ligands which carry positive charge . Example : NH2–NH3+ (Hydrazinium ion) , NO+ (Nitrosonium ion) , NO2+ (Nitronium ion)
Types of Ligands on the Basis of Denticity
The number of coordinating or ligating groups present in a ligand is called the denticity of that ligand.
Monodentate or unidentate ligands : If only one donor atom is present in a molecule which can coordinate , then it is called unidentate ligand . Example : NH3 (Ammonia), H2O (water ), CN– (Cyanide ion)
Bidentate ligands : If two donor atoms are present in a molecule which can coordinate , then it is called bidentate ligand . Example : Ethylenediamine
Tridentate ligands : The ligands which contain three donor atoms. Example : Diethylene triamine
Hexadentate ligands: If six donor atoms are present in a molecule which can coordinate , then it is called hexadentate ligand . Example : Ethylenediamine tetraacetate ion
2) Ambidentate Ligands
Unidentate ligands containing more than one donor atom but in forming complexes only one donor atom is linked to the metal ion , such ligands are called Ambidentate ligands.
3) Chelating Ligands
The bidentate or Polydentate ligand which is attached to central metal atom or ion and form a cyclic ring structure is called chelating ligand and these complexes are called chelates . This property is called chelation .
Characteristics of Chelates :
- Chelating ligands form more stable complexes than the unidentate analogs. This is called chelating effect.
- Greater the denticity of the ligand , more stable is the complex (chelate) formed.
- Ligands with large groups form unstable rings than the ligands with smaller groups due to steric hinderance .
- Chelating ligands which do not contain double bonds, e.g., ethylenediamine form five membered stable ring . The chelating ligands such as acetylacetone form six membered stable ring complex.
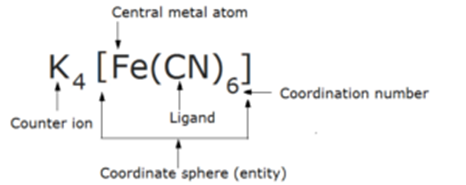
4) Coordination Number
The total number of unidentate ligands attached to the central metal ion through coordinate bonds is called the coordination number of the metal ion . In other words, coordination number may be defined as the number of atoms of the ligands which are directly bonded to the central metal atom or ion or coordination number may be defined as the number of coordinate bonds formed with the central metal atom by the ligands.
Coordination number = number of ligands x Denticity
In [Ag(CN)2]– : Coordination number of Ag is 2
In [Fe(C2O4)3]2- : Coordination of Fe is 6 since C2O4 2– is a bidentate ligand.
Note : Coordination Number of the central metal atom is determined by the number of sigma bonds formed by the ligands with central metal atom / ion . Pi bonds if formed between the ligand and the central atom / ion are not considered for this purpose.
5) Coordination Sphere or Coordination Entity and Counter Ion
The central atom and the ligands which are directly attached to it are enclosed in square brackets and are collectively termed as the coordination sphere or coordination entity.
The ligands and the metal atom inside the square bracket behave as single constituent unit . The ionizable groups are written outside the brackets and are called as counter ions.
6) Oxidation Number or Oxidation States
The oxidation number of the central atom is defined as the charge that it carries as calculated by assigning appropriate charges to the ligands and equating the sum of the charges on the central atom and the ligands equal to the charge on the coordination sphere.
Oxidation number of Fe in [Fe(CN)6]3– is +3 but the coordination number is 6 .
Oxidation number of Cu in [Cu(NH3)4]SO4 :Sulphate ion carries charge = -2 . As the complex is neutral , charge on complex ion should be = +2 . As (NH3) carries no charge , therefore charge on copper = +2, i.e., oxidation number of Cu = +2. We write it as Cu(II).
Oxidation number of Fe in K3[Fe(C2O4)3] : As each K+ ion carries charge = +1 , charge on 3K+ ions = + 3. Hence , charge on complex ion = -3. As each oxalate ion C2O4 2- has charge = -2 , charge on three C2O4 2- = -6 . Therefore, charge on Fe should be = +3, i.e., oxidation number of Fe in the given complex = +3. We write it as Fe(III).
Oxidation number of Ni in [Ni(CO)4] : Total charge on complex = 0 . As CO carries no charge , charge on Ni should be =0, i.e., oxidation number of Ni in the given complex = 0. We write it as Ni(0).
7) Homoleptic and Heteroleptic Complexes
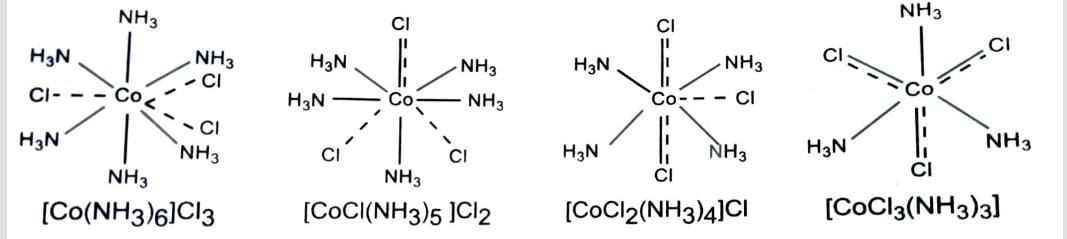
Complex in which metal atom or ion is linked to only one type of ligands are called homoleptic complexes, e.g., [Co(NH3)6]3+ . The complexes in which the metal atom or ion is linked to more than one kind of ligands are called heteroleptic complexes, e.g., [Co(NH3)4Cl2]+.
Formula Writing and Nomenclature of Coordination Compounds
1) Order of Naming Ions
The positive ion (cation) whether simple or complex is named first followed by the negative ion (anion) . The name is started with a small letter and complex part is written as one word.
2) Naming of Ligands
Anionic ligands ending with -ide are named by replacing -ide with suffix -o or -e by -o.
| Anion | Symbol | Name as ligand |
| Fluoride | F– | Flourido or fluoro |
| Chloride | Cl– | Chlorido or chloro |
| Bromide | Br– | Bromido or Bromo |
| Cyanide | CN– | Cyanido or Cyano |
| Isocyanide | NC– | Isocyanido or Isocyano |
| Oxide | O2- | Oxido or oxo |
| Hydroxide | OH– | Hydroxido or Hydroxo |
| Sulphide | S2- | Sulphido or sulpho |
| Amide | NH2– | Amido |
| imide | NH2- | imido |
| Phosphide | P3- | phosphido |
| Peroxide | O22- | peroxido or peroxo |
Ligands whose name ends with -ite or -ate become -ito or -ato, i.e., by replacing the ending -e with -o.
| Carbonate | CO32- | carbonato |
| Oxalate | C2O42- | oxalato |
| Sulphate | SO42- | sulphato |
| Nitrate | NO3– | nitrato |
| Nitro | NO2– | nitro or nitrito-N |
| Nitrite | ONO– | nitrito |
| Cyanate | OCN– | cyanato |
| Isocyanate | NCO– | isocyanato |
| Sulphite | SO32- | sulphito |
| Sulphate | SO42- | sulphato |
| Ethylenediaminetetraacetate | 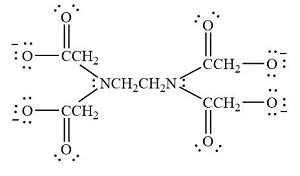 |
ethylenediaminetetraacetato |
| Acetate | CH3COO– | acetato |
| Thiosulphate | S2O32- | thiosulphato |
| Glycinate | CH2(NH2)COO– | glycinato |
| Thiocyanate | SCN– | thiocyanato |
| Isothiocyanate | NCS– | isothiocyanato |
| Acetyl acetonate |  |
acetylacetonato |
Neutral ligands are given the same name as neutral molecules.
| NH3 | ammine (not amine) |
| H2O | aqua |
| PH3 | phosphine |
| CO | carbonyl |
| CS | thiocarbonyl |
| NO | nitrosyl |
| (C6H5)3P | triphenyl |
| H2NCSNH2 | thiourea |
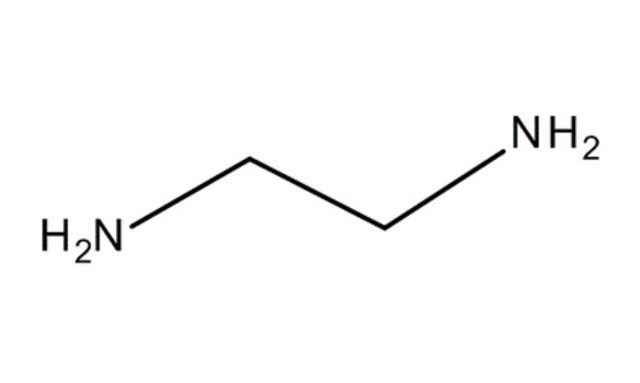 |
ethylenediamine |
| CH3NH2 | methylamine |
3) Numerical Prefixes to Indicate Number of Ligands
If there are several ligands of the same type , the prefixes like di , tri , tetra , penta and hexa are used to indicate the number of ligands of that type.
When the name of polydentate ligand includes a number, e.g., dipyridyl or ethylenediamine, then bis, tris , tetrakis, pentakis, hexakis, etc, are used instead of di- , tri- , tetra- , penta, hexa and the name of the group is placed within brackets without any hyphens.
4) Order of Naming of Ligands
All ligands whether negative , neutral or positive are named first in the alphabetical order followed by the name of the metal atom / ion. The prefixes di, tri etc. are not considered while determining the alphabetical order.
5) Naming of the Complex Ion and Ending of the Central Metal Atom
If the complex ion is a cation , the name of the central metal ion is written as such followed by its oxidation state indicated by roman numeral.
If the complex ion is anion , name of central metal atom is made to end in —ate followed by the oxidation number in bracket without any species between them.
6) Naming of Bridging Groups in Polynuclear Coordination Compounds
If the complex contain two or more metal ions, it is termed polynuclear. The monodentate ligands that link the two metal atoms simultaneously are called bridging ligands or bridge groups and are seperated from rest of the complex by hyphens and denoted by the prefix μ.
7) Naming of the Coordination Compounds Containing Metal – Metal Bonding
If the complex entity contains one metal- metal bond, the prefix bi is used with the name of metal atom.
| Complex Compound | Name |
Coordination compound containing cationic complex ion
| [Co(NH3)6]Cl3 | hexaamminecobalt (III) chloride |
| [Cr(H2O)4Cl2]NO3 | tetraaquadichloridochromium (III) nitrate |
| [Co(NH3)4Cl(NO2)] NO3 | tetraamminechloridonitrocobalt (III) nitrate |
| [CoCl2(en)2]SO4 | dichloridobis(ethane-1,2-diamine)cobalt (IV) sulphate |
| [Co(NH3)6]ClSO4 | hexaamminecobalt (III) chloride sulphate |
Coordination compound containing anionic complex ion
| K3 [Fe(C2O4)3] | potassium trioxalatoferrate (III) |
| K3 [Co(CN)5(NO)] | potassium pentacyanonitrosylcobaltate (II) |
| Na2 [CrF4O] | sodium tetrafluoridooxochromate (IV) |
| K4[Ni(CN)4] | potassium tetracyanonickelate (0) |
| Fe4 [Fe(CN)6]3 | ferric hexacyanoferrate (II) |
Coordination compounds containing complex cationic and anionic ions
| [Pt(NH3)4Cl2] [PtCl4] | tetraamminedichloridoplatinum (IV) tetrachloridoplatinate (II) |
| [Ag(NH3)2] [Ag(CN)2] | diammine silver (I) dicyanido argentate (I) |
| [Pt(py)4] [PtCl4] | tetrapyridine platinum (II) tetrachlorido platinate (II) |
Non – ionic coordination compounds
| [Co (NH3)3 (NO2)3] | triamminetrinitrocobalt (III) |
| [Pt (NH3)2 Cl2] | diamminedichloridoplatinum (II) |
| [Ni(CO)4] | tetracarbonylnickel (0) |
| [Ni(dmg)2] | bis(dimethylglyoximato) nickel (II) |
Writing Formula of a Complex when Name is Given
- The simple ion (counter ion) whether cation or anion behaves like free ion and carries usual charge as one radical.
- Charge on the complex ion (coordination entity) is computed on the basis that it is equal to the charge on the central metal atom (same as oxidation state) plus the total charge carried by the coordinated ligands. If the charge on complex ion is x, then x = oxidation state of metal + charge on ligands (with signs)
- Since a molecule of the coordination compound as a whole is neutral, total positive charge must be equal to the total negative charge. The cations and anions are, therefore, multiplied by such constants so that the charge become equal. These constant give the number of each type of ions.
Question : Formulate the compound tetraammineaquachloridocobalt (III) chloride.
Solution : Anion (simple ion) =Cl–
For the coordination entity, writing first the symbol for metal atom and then ligands in alphabetical order, we get; Cation (Complex ion) = [Co (NH3)4 (H2O) Cl]x where x is charge on complex ion
x= oxidation state of cobalt + charge on ligands = +3 +0 +0 +(-1) = +2
Charge on one anion = -1
Charge on complex cation = +2
Clearly, two anions will neutralise the charge of cation
Hence, formula of the given compound is [Co (NH3)4 (H2O) Cl] Cl2
Isomerism in Coordination Compounds
Two or more substances having the same molecular formula but different structural or spatial arrangements are called isomers and the phenomenon is called isomerism.
Coordination compound show two main types of isomerism
A) Structural isomerism B) Stereo isomerism or space isomerism
A) Structural Isomerism
It arises due to difference in structures of coordination compounds. It is of following types:
1) Ionisation Isomerism
Compounds which give different ions in solution although they have same compositions are called ionisation isomers. Example : [Pt (NH3)4 (OH)2] SO4 and [Pt (NH3)4 (SO4) ] (OH)2
2) Solvate / Hydrate Isomerism
Compounds which have the same composition but differ in the number of solvent molecules present as ligand (in coordination sphere) and as free solvent molecule (present outside the coordination sphere) in the crystal lattice are called solvent isomers. If water is solvent, these are called hydrate isomers.
Example : [Co (NH3)4 (H2O) Cl ] Cl2 and [Co (NH3)4 Cl2 ] Cl. H2O
3) Linkage Isomerism
Compounds which have same composition but differ only in point of attachment of ambidentate ligand . Example : [Co (NH3)5 (ONO)]2+ and [Co (NH3)5 (NO2)]2+
4) Coordination Isomerism
This type of isomerism is possible when both positive and negative ions of a salt are complex ions and two isomers differ in distribution of ligands in cation and anion.
Example: [Co (NH3)6 ] [Cr (CN)6] and [Cr (NH3)6 ] [Co (CN)6]
B) Stereo – Isomerism
Stereo or space isomerism arises on account of the different positions and arrangements of ligands in space around the metal ion. It is of two types.
1. Geometrical Isomerism
When two identical groups (ligands) occupy adjacent position , the isomer is called cis and when arranged opposite to one another, the isomer is called trans.
This isomerism is not possible for complexes with coordination no 2 and 3 and tetrahedral complexes with coordination no 4 since in all these cases, all four positions are equivalent.
It occurs in square planar compound of type [M A2 B2], [M A2 BC] , [M (AB)2] and octahedral complex of type [M A4 B2] , [M (AA)2 B2] , [M A3 B3]
2) Optical Isomerism
This is shown by complexes whose mirror images are non- superimposable. Such complexes are called optical isomers . It is shown by octahedral complexes and exists in two forms laevo and dextro. The isomer which rotates the plane polarised light toward right is called dextro rotatory (d-) and the other rotates towards left is called laevo rotatory (l-) . It is shown by the complexes of the type [M(AA)3], [M (AA)2 B2], [M (AA) B2 C2] .
Note : Only cis- form shows optical isomerism but not trans- form.
Werner’s Theory of Coordination Compounds
Alfred Werner prepared a theory to explain the nature of bonding in coordination compound called Werner’s coordination theory.
Postulates:
- The central metal ion in coordination compound consists of two types of valencies : Primary valency and secondary valency.
- Primary valencies are those which a metal exhibits in the formation of its simple salts. It is normally ionisable and is referred to as oxidation state.
- Secondary valencies are those which a metal atom or cation exercises towards neutral molecules or negative groups in the formation of its complex ions.
- Each metal cation has a fixed number of secondary valencies. This secondary valency is also termed as coordination number.
- Each metal atom has a tendency to satisfy both its primary and secondary valencies.
- Primary valencies are satisfied by negative ions whereas secondary valencies are satisfied by negative ions or neutral molecules.
- The secondary valencies are responsible for isomerism in the complex compounds.
- The secondary valencies are directional and primary valencies are non- directional.
- If metal has six secondary valencies then coordinate compound has octahedral structure and if metal has four secondary valencies then structure of complex will be either tetrahedral or square planar.
Structure of Complexes
Taking the example of complexes formed between CoCl3 and NH3, the ionizable chloride ions are formed by precipitation with AgNO3. They are linked by primary valencies. The remaining Cl atoms and NH3 molecules are present around the central metal atom in such directions that repulsion between them is minimum thereby giving a definite shape. These atoms and groups are linked by secondary valencies.
Representing the primary valencies by dotted lines and secondary valencies by solid lines.
Valence Bond Theory (VBT)
This theory was extended to coordination compound by Pauling in 1931. The following are the main postulates of this theory:
- Metal-ligand bond are formed by the donation of pairs of electrons by the ligands to the metal atom/ ion.
- In order to accomodate these electrons, the metal ion must possess requisite number of valence orbitals of equal energy. These orbitals are obtained by hybridisation of the orbitals of the metal atom to give a set of hybrid orbitals of equal energy.
- Sometimes , unpaired (n-1)d electrons pair up as fully as possible prior to hybridisation thus making some (n-1)d orbitals vacant. The central atom then makes available the number of empty orbitals equal to its coordination number for formation of coordinate bonds with suitable ligand orbitals.
- With approach of ligands, metal ligand bonds are then formed by the overlap off these orbitals with those of ligands. These bonds are of equal strength and directional in nature.
- Octahedral (due to d2sp3 or sp3d2 hybridisation) , square planar (due to dsp2) and tetrahedral (sp3) complexes are formed.
Note : CN– , CO, NO2– are strong ligands and as they approach the metal ion , the electrons must pair up.
Complexes with Coordination Number 4
Complexes with coordination number 4 are either tetrahedral or square planar.
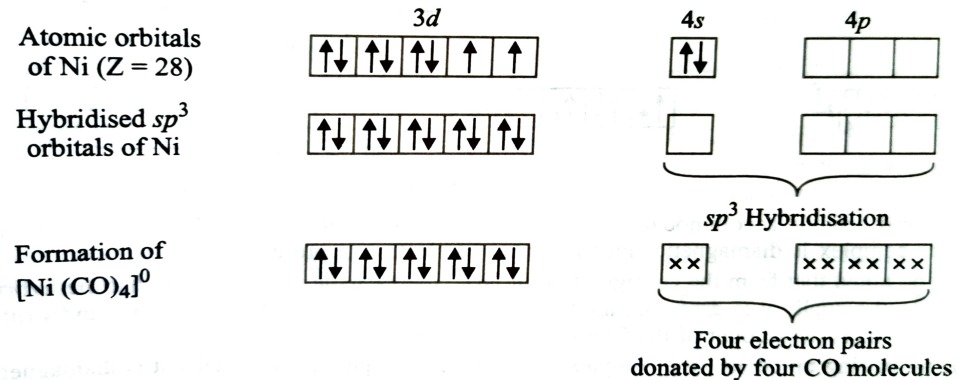
Formation of [Ni(CO)4]
Oxidation state of nickel in this complex is 0.
Its electronic configuration is [Ar] 3d8 4s2
Thus the resulting complex is diamagnetic. Structure of complex will be tetrahedral due to sp3 hybridisation.
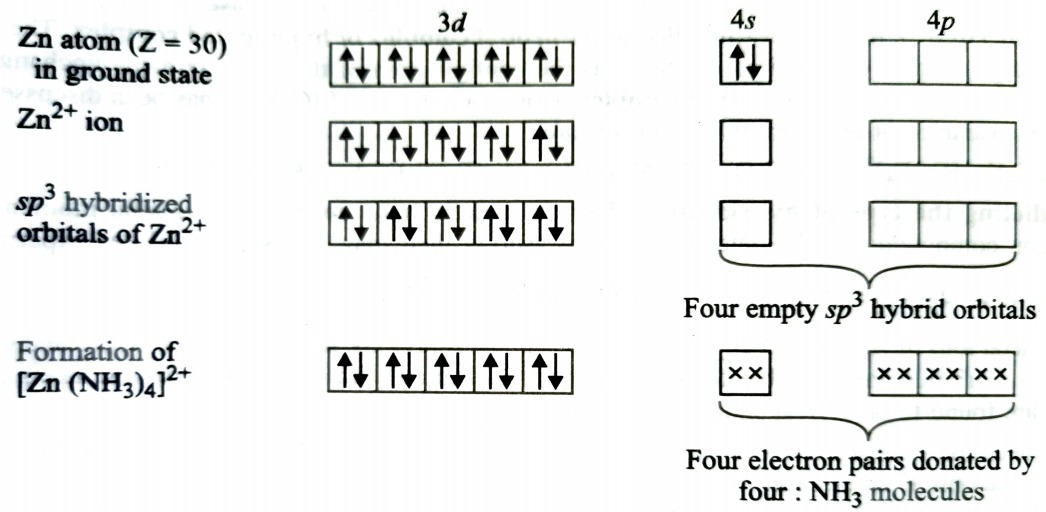
Formation of [Zn(NH3)4]2+
Complexes of Zn2+ are invariably tetrahedral because they involve sp3 hybridisation. The complex will be diamagnetic as there are no unpaired electron.
In the above case, 3d orbitals are fully occpied therefore, they cannot participate in hybridisation. The four sp3 hybrid orbitals accomodate four pair of electrons from ammonia molecules.
Complexes with Coordination Number 6
Octahedral complexes are of two types :
| Inner Orbital Octahedral Complexes or Low Spin Complexes | Outer Orbital Octahedral Complexes or High Spin Complexes |
| It involves d2 sp3 hybridisation. | It involves sp3 d2 hybridisation. |
| These are formed by strong ligands. | These are formed by weak ligands. |
| These complexes possess less number of unpaired electrons. | These complexes possess greater number of unpaired electrons. |
| They show low or no magnetic moment. | They show high magnetic moment. |
Formation of [Fe (CN)6 ] 4-
Oxidation state of Fe is +2. The resulting complex ion is inner orbital octahedral and diamagnetic as it does not have unpaired electron.
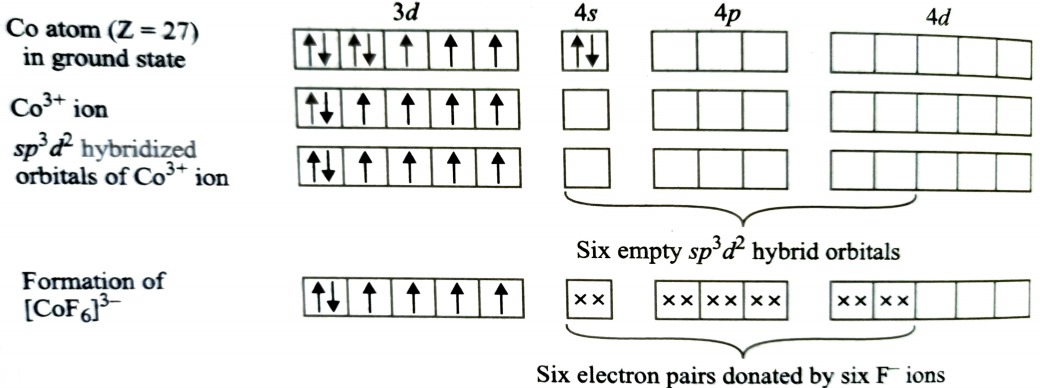
Formation of [CoF6]3-
Oxidation state of cobalt in this complex is +3. F- ion is a weak ligand and is unable to pair up the unpaired electrons of 3d orbitals. It is highly paramagnetic due to presence of 4 unpaired electrons. The resulting complex is called outer orbital octahedral complex.
Limitation of VBT
- It could not give any satisfactorily explanation for the colour of the complexes.
- It does not distinguish between weak and strong ligands.
- In a number of cases, the experimentally observed values of magnetic moment do not easily coinicide with values calculated from valence bond theory.
- It cannot explain why some complexes of metal ion in a particular oxidation state are low spin while some other complexes of the same metal ion in same oxidation state are high spin.
- The magnetic behaviour predicted by valence bond theory is sometimes misleading.
- It is based on the number of assumptions.
- It fails to explain the relative energies of different shapes.
Crystal Field Theory
The main points of this theory are as follows:
- Crystal field theory considers the bond to be purely ionic arising purely from electrostatic attraction between the metal ion and the ligands.
- It treats each ligand as a point of negative charge . The arrangement of the ligands around the central metal ion is such that repulsion between these negative points are minimum.
- In a free transition – metal ion, all the five d orbitals have equal energies, i.e., they are degenerate. This degeneracy is maintained if negative charges present around the central metal atom / ion form a spherically symmetrical field.
- Under the influence of ligand field, degeneracy of d orbital is destroyed and it splits into two or more energy levels.
- The extent of splitting depend upon the strength of ligand. A strong ligand causes greater splitting while a weak ligand causes a smaller splitting.
The difference of energy between the two sets of d orbital (in octahedral complexes) is called crystal field splitting energy or Δo .
Crystal Field Theory for Octahedral Complexes
- As the ligands approach the metal ion, there is repulsion between ligand and the d orbitals.
- d x2 – y 2 and d z2 orbitals have lobes along the axes whereas d xy, dxz, dyz orbitals lie between the axes.
- As a result , repulsion between the ligands and the d x2 – y 2 and d z2 orbitals are greater than repulsion between the ligands and remaining three d- orbitals.
- Thus energies of d xy, dxz, dyz orbitals are lower than those of d x2 – y 2 and d z2 orbitals . Hence former three orbitals of lower energy are called t2g orbitals and the latter two of high energy are called eg orbitals.
Factors affecting magnitude of CFSE : The ligands with smaller size, large negative charge, with good donor and acceptor properties will give large field splitting.
The increasing order of the values Δo is as follows : I–< Br– < SCN– < Cl– < S2- < F– < OH– < C2O42- < H2O < NCS– < EDTA4- < NH3 < en < CN < CO . This series is called spectrochemical series.
Calculation of CFSE
Each electron occupying t2g orbital results in lowering of energy by -0.40Δo. Similarily each electron occupying eg orbital results in increase of energy by +0.60Δo.
CFSE = ( -0.40x + 0.60y ) Δo where x is number of electrons occupying t2g orbital and y is electrons occupying eg orbital. [ 1Δo = 10Dq]
For d1 configuration t12g ; CFSE = -0.4 x 1 = -0.4 Δo
For d2 configuration t22g ; CFSE = -0.4 x 2 = -0.8 Δo
For d3 configuration t32g ; CFSE = -0.4 x 3 = -1.2 Δo
For d8 configuration t62g eg2; CFSE = -0.4 x 6 + 0.6 x 2 = -1.2 Δo
For d9 configuration t62g eg3; CFSE = -0.4 x 6 + 0.6 x 3 = -0.6 Δo
For d10 configuration t62g eg4; CFSE = -0.4 x 6 + 0.6 x 4 = 0 Δo
Properties of the Complexes by Crystal Field Theory
1) High spin states and low spin states
For d4 to d7 configuration depends upon high spin state or low spin state
The value of Δo is usually compared with the energy required for electron pairing in a single orbital. This energy isrepresented by P and is called pairing energy.
If Δo > P , electron will pair and we get low spin complexes. Ligands for which Δo > P are known is strong field ligands.
If Δo < P , electron will not pair and we get high spin complexes. Ligands for which Δo < P are known is weak field ligands.
Note : Strong Field ligands → High Δo value → low spin complexes
Weak Filed Ligands → Low Δo value → High spin complexes
2) Magnetism
The low spin complexes have lesser no of unpaired electrons. So have low value of magnetic moment, so mostly are diamagnetic.
3) Colour
Different complexes exhibit different colours when either the metal ion is different or the metal ion is ame but ligands attached to it are different.
In absence of ligand , crystal field splitting does not occur and hence substance is colourless.
Stability of Coordination Compounds
Question : What is meant by stability of compound in solution ? State the factors which affect the stability of complexes?
Answer : Stability of complex refers to the degree of association between two species involved in state of equilibrium and stability of compound is measured in terms of stability constant . Larger the value of stability complex , higher is the stability.
Factors affecting the stability of complex ion are as follows :
- Charge on the central metal atom : Greater the charge on central metal atom, greater is the stability of the complex ion.
- Basic Nature of the ligand : greater the basic strength of the ligand, grater is the stability of the complex.
- Presence of chelate rings: Formation of chelate ring increases the stability of the complex. The stabilisation due to chelation is called chelate effect.
# Coordination Compounds Class 12 Notes
# Coordination Compounds Class 12 Notes in Detail
# Coordination Compounds Class 12 Notes with NCERT Solutions
# Coordination Compounds Class 12 Notes with Solutions
# Coordination Compounds Class 12 Notes elaborated
# Coordination Compounds Class 12 Notes
Do share the post if you liked Coordination Compounds Class 12 Notes. For more updates, keep logging on BrainyLads