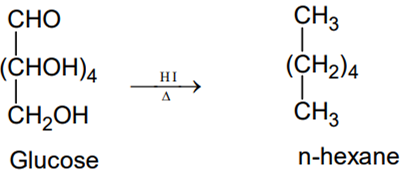Biomolecules Class 12 | Chapter 14 | Chemistry | Notes | CBSE |
Table of Contents
Biomolecules Class 12 | Chapter 14 | Chemistry | Notes | CBSE |
Biomolecules
Biomolecules Class 12 : Biomolecules may be defined as complex lifeless chemical substance which form the basis of life, i.e., they not only build up living creatures but are also responsible for their growth , maintenance and ability to reproduce. Living system is composed of non-living atoms and molecules.
Carbohydrates
Carbohydrates are defined as polyhydroxy aldehyde or ketone or substances which give these on hydrolysis.
- Carbohydrates are also known as Saccharides.
- Carbohydrates which are sweet in taste are called sugars.
- Sugar present in home is named as Sucrose.
- Sugar present in milk is known as Lactose.
Classification of Carbohydrate on the basis of their Behaviour towards Hydrolysis
1) Monnosacharides
These are carbohydrates which cannot be hydrolysed further to give simple unit of polyhydroxy aldehyde or ketone .
2) Oligosachharides
These are carbohydrates which on hydrolysis give 2-10 molecules of monnosacharides . Depending upon the number of monosaccharides molecules they actually obtained upon hydrolysis , they are further classified as di , tri , tetrasachharides, etc
- Disaccharides : Carbohydrates which upon hydrolysis give two molecule of same or different monosaccharides are called disaccharides. Example : Sucrose , Maltose , Lactose
- Trisachharides : Carbohydrates which upon hydrolysis give three molecule of same or different monosaccharides are called trisaccharides. Example : Raffinose
- Tetrasaccharides: Carbohydrates which upon hydrolysis give four molecule of same or different monosaccharides are called tetrasaccharides. Example : Stachyrose
3)Polysaccharides
Carbohydrates which upon hydrolysis give a large number of monosachharide molecules are called polysachharides . Example : Starch , Cellulose , Glycogen
Classification of Carbohydrate on the Basis of their Nature
1)Reducing Sugar
All those carbohydrate which contain aldehydic or ketonic group in the hemiacetal or hemiketal form and reduce Tollens’s reagent or Fehling ‘s solution are called reducing carbohydrates or sugars.
2)Non-Reducing Sugar
All those carbohydrate which do not reduce Tollen’s reagent or Fehling solution are called non reducing carbohydrates or sugar. Also; In disachharides if aldehydic or ketonic group is bonded , these are non reducing group. Example : Sucrose
Monosaccharides
Classification on basis of Carbonyl Functional Group
- Aldose : Monosachharide containing an aldehydic group (—CHO ) are called aldose.
- Ketose : Monosachharide containing a keto ( > C = O) group are called ketose .
Glucose
- Glucose occur in nature in free as well as in combined form.
- It occur in large quantities in grape and hence also called grape sugar.
Preparation of Glucose
i)From Sucrose (Cane Sugar) : When sucrose is hydrolysed by boiling with dil. HCl in alcoholic solution , an equimolar mixture of glucose and fructose are obtained .
C12H22O11 (sucrose) + H2O → C6H12O6 (Glucose ) + C6H12O6 (Fructose)
ii) From Starch : Commercially , Glucose is obtained by hydrolysis of starch by boiling it with dil. H2SO4 under pressure.
(C6H10O5)n (starch) + n H2O → n C6H12O6 (Glucose )
Structure of Glucose
- Glucose is an aldohexose and is also called dextrose .
- The molecular formula of glucose is C6H12O6.
- Open chain structure of Glucose was proposed by Baeyer.
- On prolonged heating with HI, it forms n hexane .
- On oxidation with Bromine water or Tollen’s reagent, it gives gluconic acid
- Glucose reacts with hydroxylamine to form an oxime
- Glucose adds a molecule of hydrogen cyanide to form cyanohydrin.
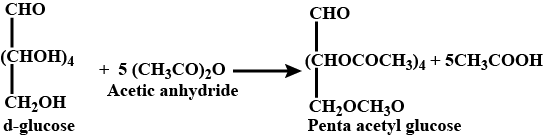
- On acetylation with acetic anhydride, glucose gives a pentaacetate. This confirms the presence of five —OH groups.
- On oxidation with nitric acid, both glucose and gluconic acid give the same dicarboxylic acid , saccharic acid or glucaric acid (older name glucaric acid)
- On reduction with sodium amlgam , glucose yields sorbitol , a hexahydric alcohol.
- Since the molecule of glucose has 4 chiral carbon , Thus it exists in 24 = 16 isomers .
- The exact arrangement of different –OH group was determined by Emil Fischer
- The correct name of Glucose is D-(+)-Glucose . Since glucose is optically active , its enantiomer is called L-(-)-glucose .
- The letter D and L used before the name of glucose represent the configuration of OH group at penultimate carbon atom whereas algebraic signs + and – refer to sign of optical rotation.
Note : ‘D’ and ‘L’ have no relation with sign of optical rotation
- The simplest carbohydrate, glyceraldehyde contain one chiral atom and hence exist in two enantiomeric forms.
- All those compound which can be chemically correlated to (+)-isomer of glyceraldehyde are assigned D-configuration wheras all those which can be chemically correlated to (-)-isomer of glyceraldehyde are assigned L configuration.
Reactions of Glucose which could not be explained by its open structure :
- D-(+)-Glucose does not undergo certain characteristic reaction of aldehyde . For example , glucose does not form NaHSO3 addition product , aldehyde ammonia adduct , 2,4-DNP derivative and does not respond to Schiff’s reagent test.
- Glucose reacts with NH2OH to form an oxime but glucose pentaacetate does not . This implies that aldehyde group is absent in glucose pentaacetate.
- D-(+)-Glucose exists in two stereoisomeric forms ,i.e., α-glucose and β-glucose . The α form is obtained by crystallisation from concentrated solution of glucose at 303 K while β form is obtained by crystallisation form hot and saturated and aqueous solution at 371K.
- Above reaction of glucose are not explained by its open structure since the –CHO group of glucose has interacted with C5—OH group to form an hemiacetal cyclic structure . This explains the absence of –CHO group
- Such a pair of stereoisomers which differ in configuration only around C 1 are called anomers and the C 1 carbon is called the anomeric carbon or the glycosidic carbon.
Cyclic Structure of Glucose
The six membered ring containing one oxygen atom because of its resemblance with pyran is called the pyranose form . The cyclic structure is more correctly represented by Haworth.
Fructose
It is an important ketohexose . It occurs free long with glucose in honey and sweet fruit and hence named fruit sugar.
Structure of Fructose
- Its molecular formula is C6H12O6.
- It belongs to D series and is a Laevorotatory compound .
- It contain a keto group at C2 and six carbon atoms are arranged in straight chain .
- Its correct name is D-(-)-fructose.
- The five membered cyclic structure of fructose is named as Furanose with analogy to compound furan. Furan is a five membered cyclic compound with one oxygen and four carbon atoms .
Haworth structure of two anomers of fructose
Disaccharides
Carbohydrates which upon hydrolysis yield two molecule of same or different monosaccharides.
The two monosaccharide units are joined together through an oxide linkage formed by loss of water molecule . Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage.
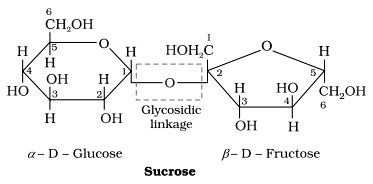
i) Sucrose
- It is colourless , crystalline , water soluble sweet substance .
- On hydrolysis with dilute acid , it gives an equimolar mixture of D-(+)-glucose and D-(-)-fructose .
C12H22O11 (sucrose) + H2O → C6H12O6 ( D-(+)-Glucose ) + C6H12O6 ( D-(-)-Fructose)
- Sucrose is a non reducing sugar .
- Glycosidic linkage is between C1 of α-Glucose and C2 of β-fructose.
- Sucrose is dextrorotatory bu after hydrolysis gives a mixture of dextrorotatory glucose and laevorotatory fructose . Since the laevorotation of fructose is much more than dextorotation of glucose ; therefore resulting mixture become laevorotatory .
- Since there is a change in signof rotation , product is named as invert sugar.
ii) Maltose
- It is reducing sugar.
- Hydrolysis of one mole of maltose yields two moles of D-glucose.
- C1 of one glucose unit is linked to C4 of other .
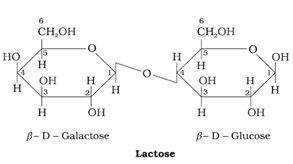
iii) Lactose
- It occurs in milk and that’s why called as milk sugar.
- It is a reducing sugar.
- C1 of galactose unit is linked to C4 of glucose unit.
Polysaccharides
Polysaccharides are formed when large number of monosaccharide joined togeter by glycosidic linkage.
i) Starch
- It is white amorphous powder sparingly soluble in water.
- On hydrolysis with dilute mineral acid , starch breaks first into smaller molecules , then to maltose and finally to D-glucose.
- Starch is non reducing saccharide .
- It is a polymer of α -glucose and consists of two components – a water soluble component called Amylose and a water insoluble component called Amyloceptin.
- Amylose is a linear polymer of α-D-glucose having helical structure in which C1 of one glucose unit is attached to C4 of other.
- Amyloceptin is highly branched polymer in which chain is formed between C1-C4 glycosidic linkage whereas branching occurs by C1-C6 glycosidic linkage.
ii) Cellulose
- It is chief structural material of cell wall of plants.
- It is also non-reducing sugar.
- It is linear condensation of polymer of β-D-glucose in which C1 of one glucose is connected to C4 of other through glycosidic linkage.
iii) Glycogen
- It is also called animal starch because its structure is similar to that of amyloceptin.
- When body needs glucose ; enzyme breaks the glycogen down to glucose .
- It is more highly branched than amyloceptin.
Amino Acids
Amino acid contain amino and carboxyl as functional group.
Depending upon relative position of NH2 group wrt COOH group they are classified as α , β ,γ , δ . Only α amino acid are the building block of protein.
Amino acids are colourless , crystalline solids. These are water soluble due to intermolecular H-Bonding with water molecule.
Neutral , Acidic and Basic amino acids :
- In Acidic amino acids , carboxyl groups are more than the amino group . Example : Aspartic acid , Glutanic acid.
- In Basic amino acids , amino group are in excess than carboxyl group . Example : Lysine , Arginine
- In Neutral amino acid , amino group and carboxyl group are equal in number . Example : Alanine , Leucine
Essential and non-essential amino acids :
- Those amino acid which cannot be synthesized in body but must be obtained through diet are known as essential or indispensable amino acids . Example : Valine , Lysine , Histidine
- Those amino acid which can be synthesized by the body are called Non – Essential amino acids. Example: Glycine , Alanine
Zwitter ionic form
In aqueous solution , carboxyl group can loose a proton and amino acid can accept giving rise to dipolar ion called Zwitter ion .
In Zwitter ionic form , amino acids show amphoteric behaviour.
The pH at which a particular amino acid neither migrate towards anode nor towards cathode under the influence of applied electric field is called iso-electric point of amino acid.
Note : Except Glycine, all α-amino acids are optically active.
Protein
Protein may be defined as complex organic nitrogenous substances which on hydrolysis give large number of molecule of amino acids .
- Protein are polymer of α-amino acids and they are connected to each other by peptide bond or peptide linkage.
- Peptide are amide formed by the condensation of amino group of one α-amino acid with the carboxyl group of another molecule of same or different α-amino acid with the elimination of a molecule of water.
- Example : when –COOH group of glycine combines with –NH2 group of alanine , we get a a dipeptide , glycylalanine .
Classification of Protein based upon composition
| Fibrous Protein | Globular Protein |
| These protein consist of linear thread like structure which tend to lie side by side to form fibres. | The polypeptide chain in these protein is folded around itself in such a way so as to give entire protein molecule in spherical shape. |
| They are usually held together at many points by hydrogen bonds and some sulphite bonds. | They are held tightly by weak intramolecular hydrogen bonding. |
| These protein are stable to moderate changes in temperature and pH. | These are very sensitive to small changes in temperature and pH . |
| These are insoluble in water. | These are water soluble. |
| These are chief structural material of animal tissues. | These protein regulate and maintain the life cycle. |
| Example : keratin in skin , myosin in muscles | Example : Insulin , Haemoglobin , Albumins |
Structure of Proteins
1)Primary Structure of Protein
Protein may have one or more polypeptide chains. Each polypeptide chain has large number of α-Amino acids linked to one another in a specific sequence . The specific sequence in which various amino acid are linked to one another is called its primary structure.
The importance of primary structure of protein in determining its biological activity is shown by the fact that replacement of just one amino acid in the sequence of protein destroys its biological activity.
2)Secondary Structure of Protein
The conformation which the polypeptide chain assume as a result of hydrogen bonding is called the secondary structure of protein. Following two different secondary groups are possible :-
| α-Helix Structure | β-pleated Structure |
| It is formed due to regular folding of polypeptide chains. | In this structure , all peptide chains are streched to their maximum extension and then arranged side by side and form a flat sheet structure. |
| In this size of R groups is quite large. | In this size of R groups is quite small. |
| There is intramolecular hydrogen bonding . | There is intermolecular hydrogen bonding between neighbouring peptide chains . |
| Example : Keratin and Myosin in nucleus | Example : Fibrion in silk fibre. |
3)Tertiary Structure of Protein
The tertiary structure of protein represents overall folding of the polypeptide chains, i.e., further folding of the secondary structure. In other words , tertiary structure refers to the manner in the entire protein molecule folds up in the three dimensional space to produce a specific shape .
4)Quaternary Structure of Protein
The quaternary structure refers to the determination of the number of subunits and their spatial arrangement w.r.t each other in an aggregate protein molecule.
Denaturation of Protein
A protein found in biological system having unique three dimensional structure and specific biological activity is called native protein.
When a protein in its native form is subjected to a physical change like change in temperature or a chemical change like change in pH , the native conformation of molecule is disrupted . As a result , hydrogen bonds are disturbed , globules unfold and helices get uncoiled to form thread like molecule .
In other words , globular protein undergo coagulation or precipitation to give fibrous proteins . Due to coagulation the native shape of protein is destroyed and biological activity is lost . That’s why coagulated protein so formed is called denaturated protein.
Chemically , during denaturation , the secondary and tertiary structure are destroyed but primary structure remain intact.
Example : coagulation of egg white on boiling , curdling of milk.
Vitamins
- Vitamins may be defined as a group of biomolecules most of which cannot be produced by the body and must be applied in small amount in diet to perform the specific biological function for the life , growth and health of human being and animal organisms.
- Plants can synthesize all vitamins but only few are synthesize in animals .
Classification of Vitamins
1) Water Soluble Vitamins
- The vitamins which are soluble in water are called water soluble vitamins.
- These include vitamin B complex and vitamin C .
- Water soluble vitamins must be supplied regularly since they cannot be stored regularly in diet because they are regularly excreted in urine and cannot be stored.
2)Fat Soluble Vitamin
- These are oily substances not readily soluble in water. They are soluble in Fat .
- These include vitamins A, D , E and K . Excess intake of these vitamins is harmful and may cause hypervitaminoses.
Lack of a particular vitamin causes a specific deficiency disease . Multiple deficiencies caused by lack of more than one vitamin is called avitaminoses.
Nucleic Acids
Nucleus of a living cell is responsible for transmission of inherent characters called heredity .
The particles present in nucleus of cell which are responsible for heredity are called chromosomes which are made up of proteins and another type of biomolecules called nucleic acids.
Chemical Composition of Nucleic Acids
- Pentose Sugar
- Nitrogen containing heterocyclic compound also called nitrogenous bases
- Phosphoric Acid
Bases : There are two different classes of heterocyclic nitrogenous bases which have been isolated by the hydrolysis of nucleic acids.
i) Purines : Two purines which are most commonly found in nucleic acids are adenine (A) and guanine (G).
ii)Pyrimidines : Three most commonly occuring pyrimidines in nucleic acids are uracil (U) , thymine (T) and cytosine (C).
Types of Nucleic Acid
| DNA (Deoxyribonucleic acid ) | RNA (Ribonucleic acid ) |
| The sugar present in DNA is 2-deoxy D-(-)-ribose. | The sugar present in RNA is D-(-)-ribose. |
| DNA contains cytosine and thymine as pyrimidine bases and guanine and adenine as purine bases. | RNA contains cytosine and Uracil as pyrimidine bases and guanine and adenine as purine bases. |
| It chiefly occurs in nucleus of cell. | It mainly occurs in cytoplasm of the cell. |
| DNA has double stranded α-helix structure. | RNA has single stranded α-helix structure. |
| DNA has the unique property of replication . | RNA usually does not replicate. |
| It controls the transmission of hereditary effects. | It controls the synthesis of proteins. |
| It is very big polymer with high molecular mass. | It is comparatively small polymer. |
Types of RNA
1)mRNA (Messenger Ribonucleic Acid)
It constitutes about 15% of total RNA . mRNA molecule carry genetic information from DNA to ribosomes.
2)rRNA (Ribosomal Ribonucleic Acid)
It constitutes about 60-80% of total RNA . It combines with protein to form ribosomes which are intracellular structures where protein are synthesized.
3)tRNA (Transfer Ribonucleic Acid)
It is about 5% of total RNA . It carry amino acid to ribosomes to incorporate into the protein.
Nucleosides and Nucleotides
Nucleosides : A unit formed by attachment of base to 1′ position of sugar is called as nucleoside. It contains only two basic components of nucleic acids, i.e., a pentose sugar and nitrogenous base.
Nucleotides : When nucleoside is linked to phosphoric acid at 5′ position of sugar moiety , we get a nucleotide . It consists of three basic components, i.e., phosphoric acid group , a pentose sugar and a nitrogenous base .
Note : Nucleotides are joined together by phosphodiester linkage between 5′ and 3′ carbon atoms of pentose sugar.
DNA Fingerprinting
A sequence of base on DNA is unique for every person and information regarding it is called DNA fingerprinting.
Uses of DNA fingerprinting are as follows :
- In forensic Lab for identification of criminals.
- To determine paternity of an individual.
- To identify dead bodies in an accident by comparing the DNA’s of their parents or children.
- To identify racial groups to rewrite biological evolution .
# Biomolecules Class 12
# Biomolecules Class 12 Notes
# Biomolecules Class 12 NCERT Solutions
# Biomolecules Class 12 Questions
# Biomolecules Class 12 Chemistry Notes
# Biomolecules Class 12 Solutions
Do share the post if you liked Biomolecules Class 12. For more updates, keep logging on BrainyLads