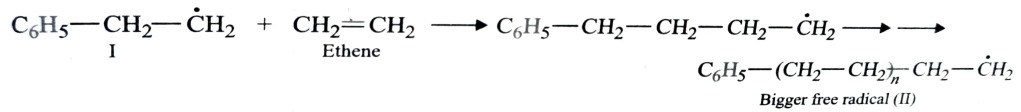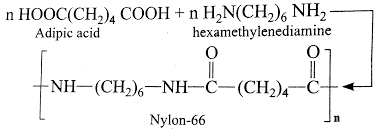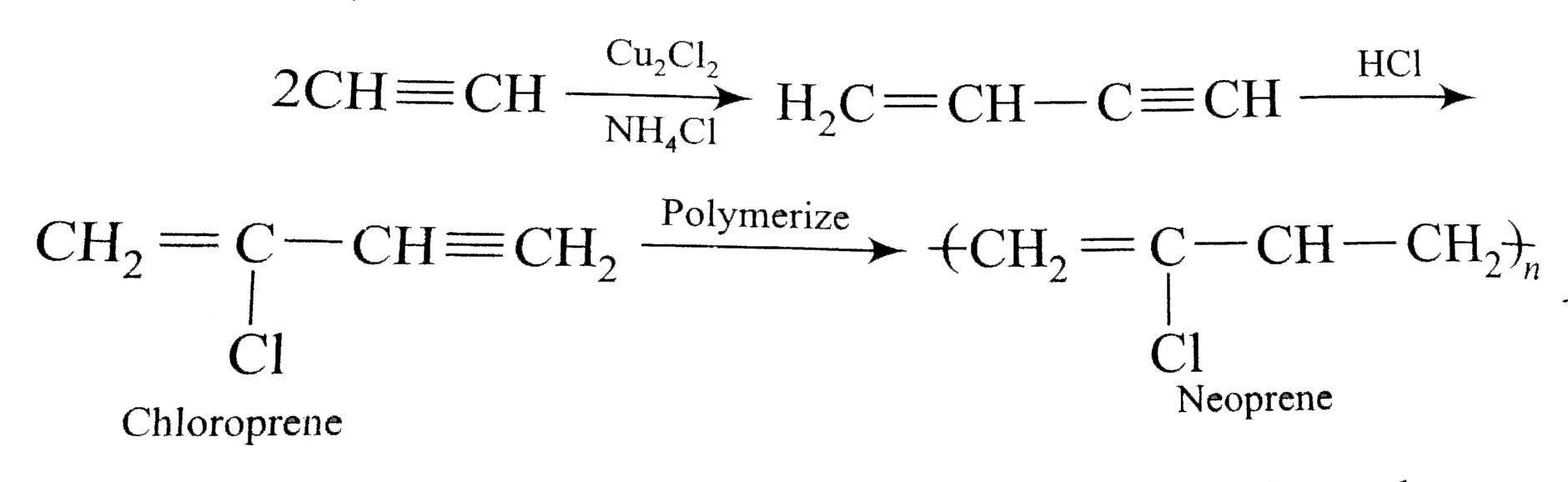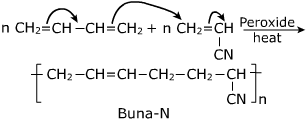Polymers Class 12 | Chapter 15 | Chemistry | CBSE |
Table of Contents
Polymers Class 12 | Chapter 15 | Chemistry | CBSE |
Polymers Class 12 Notes
Polymers Class 12 : Polymers or macromolecules are very high molecular mass (10 3 – 107 u) substances each molecule of which consists of a very large number of simple repeating structural units joined together through covalent bond in a regular fashion.
The simple and reactive molecules from which repeating structural units are derived are called monomers and the process by which these simple molecules, i.e., monomers are converted into polymers are called polymerization.
Difference between Polymers and Macromolecules
A polymer always consists of hundreds to thousands of repeating structural units but a macromolecule may or may not contain repeating structural units. For Example, proteins and nuclei acid should be regarded as macromolecules but not polymers since their molecules do not contain repeating structural units.
Note : All polymers are macromolecules but all macromolecules are not polymers.
Classification of Polymers
Classification Based upon Source
Depending upon the source from which they are obtained , polymers are broadly divvided into three classes:
Natural polymers
Polymers which are found in nature , i.e., in animals and plants are called natural polymers . Example : Protein which make much of our body , nucleic acid which control heredity , cellulose which provide food .
Semi- synthetic polymers
These are mostly derived from naturally occuring polymers by chemical modifications. . Example : cellulose on acetylation with acetic anhydride in presence of conc.H2SO4 gives cellulose diacetate which is used for making threads of acetate rayon .
Synthetic polymers
A large number of man made polymers are extensively used in daily life as well as in industry . Example : Fibres, Plastics, rubbers.
Classification Based upon Structure
Linear polymers
In these polymers , monomers are joined together to form long straight chains of polymers molecules. The various polymeric chains are then stacked over one another to give a well packed structure. They have high melting points , high densities and high tensile strength . Example : high density polythene, polyvinyl chloride nylons , poyesters etc.
Branched Chain polymers
In thiese polymers , monomer units not only combine to produce the linear chain but also form branches of different lengths along the main chain. They have lower melting point , density and tensile strength as compared to linear molecule. Example : amyloceptin , glycogen etc.
Cross Linked polymers or three dimensional network polymers
In these polymers, the inititally formed linear polymer chains are joined together to form a three dimensional structure. These polymers are hard , rigid and brittile . Example : bakelite , urea formaldehyde polymer , melamine formaldehyde polymer etc.
Classification Based upon Mode of Polymerization
Addition polymers
Addition polymers are formed by the repeated addition of large number of same or different monomers possessing double and triple bonds and the process by which addition polymers are formed is called addition polymerization . The addition polymers can be addition homopolymers or addition copolymers .
The addition polymer formed by the polymerisation of single monomeric species are known as addition homopolymers . Example : polythene , polypropene
The addition polymer formed by repeated addition of two or more type of monomeric units are called addition copolymers. Example : butyl rubber (isobutylene and isopropene) .
Condensation polymers
These are formed by repeated condensation reaction between two bifunctional or trifunctional monomer units usually with the elimination of small molecules like water , alcohol , ammonia , carbon dioxide , hydrogen chloride and the process by which condensation polymers are formed are called condensation polymerization.
Classification Based upon Molecular Forces
Elastomers
Polymers in which intermolecular force of attraction between the polymer chains are the weakest are called elastomers. They are amorphous polymers that have high degree of elasticity . These polymers consists of randomly coiled molecular chains of irregular shape having a few cross links . Weak vander waal force of attraction permit the polymer chains to be stretched , the cross link help the polymer to come back to original position . Example : vulcanized rubber
Fibres
Polymers in which intermolecular force of attraction are strongest are called fibres. These forces are either due to H-bonding or dipole – dipole interaction . Due to strong intermolecular force of attraction , they have high tensile strength and least elasticity . They have high melting point and low solubility. Example : nylons , polysters
Thermoplastics
Polymers in which intermolecular force of attraction are between elastomers and fibres are thermoplastics . They are linear or slightly branched polymer chain which are hard at room temperature , become soft and viscous on heating and again rigid on cooling . Example : teflon , polyvinyl chloride , polypropene.
Thermosetting
These are semi fluid substances with low molecular masses which when heated in a mould , undergo a permanent change in chemical composition to give a hard , infusible and insoluble mass.
Classification Based on Growth of Polymerization
Nowadays , addition polymers are referred as chain growth polymers while condensation polymers are referred as step growth polymers.
Types of Polymerization Reaction
1)Addition Polymerization or Chain Growth Polymerization
When same or different monomer molecules successively add together on a large scale to form a molecule , then this type of polymerisation is known as addition or chain growth polymerisation .
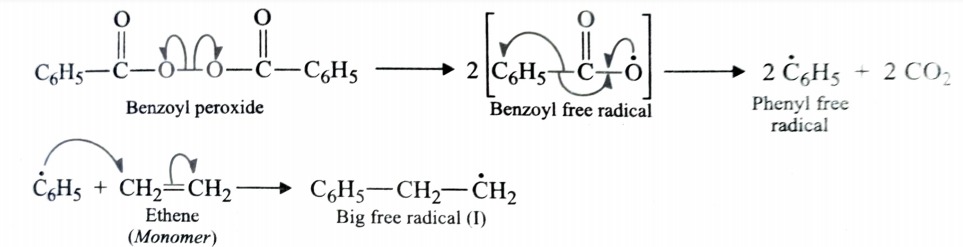
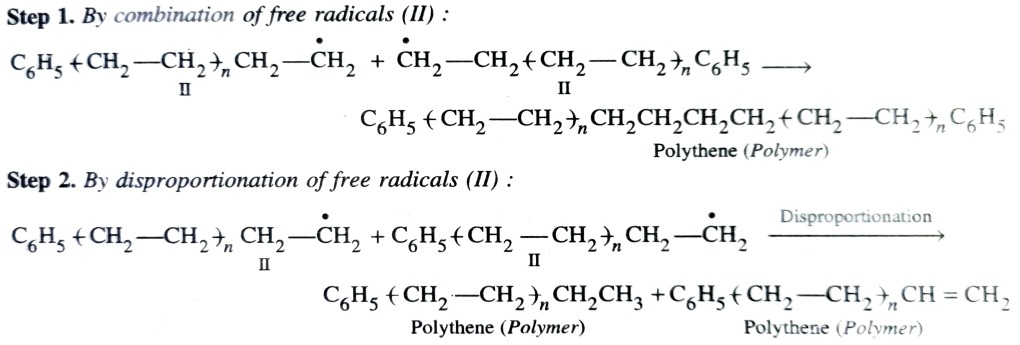
This involves free radical mechanism or ionic mechanism along which free radical one is most common mode.
Free Radical Mechanism
Chain initiating step
Chain propagating step
Chain terminating step
Some Important Addition Polymers
a)Polythene or Polyethylene
There are two types of polythene as given below :
1)Low Density Polymer
- It is a transparent polymer of moderate tensile strength and high toughness . It is chemically inert , slightly flexible and is poor conductor of electricity.
- It is manufactured by heating ethylene to 350-370K under a pressure of 1000-2000 atmosphere and in presence of trace of oxygen or peroxide.
- It is widely used as packaging material , as insulation for electrical wires and cables .
2)High Density Polymer
- It is a translucent polymer . It is also chemically inert but has greater toughness . hardness and tensile strength than low density polythene .
- It is prepared by co-ordination polymerization of ethene.
- It is used in manufacture of containers , housewares , pipes , bottles and toys.
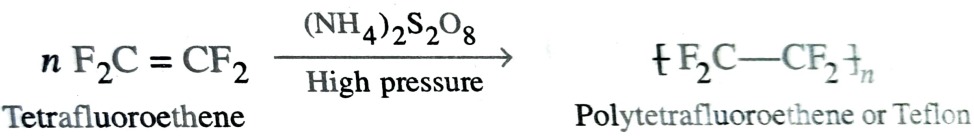
b)Polytetrafluoroethene (PTFE) or Teflon
It is manufactured by heating tetrafluoroethene in presence of peroxides or ammonium persulphate catalyst at high pressures.
Teflon is flexible and inert to solvent and to boiling acids even to aqua regia and is stable upto 598K . Because of its great chemical inertness and high thermal stability , it is used for making non stick utensils. For this purpose , a thin layer of teflon is coated on inner side of the vessel.
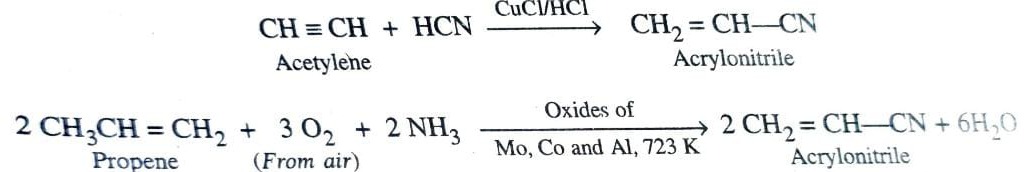
c)Polyacrylonitrile (PAN) or Orlon
It is manufactured either by addition of HCN to acetylene in presence of CuCl / HCl as catalyst or by passing a mixture of propene , ammonia and air over a catalyst consisting of mixture of oxides of molybdenum , cobalt and aluminium at 723K .
2) Condensation Polymerization or Step Growth Polymerization
It occurs in steps involving loss of small molecules like water , alcohol etc.
Some Important Condensation Polymer
a)Polyamides
Polymers which have amide linkages are called polyamides. These polymers are commonly called nylons. These are prepared by condensation polymerization of dibasic acids with diamines or their equivalents.
Preparation of Nylon 6,6 : It is manufactured by condensation polymerization of adipic acid and hexamethylenediamine. The acid and amine first react to form salt when heated to 525K under pressure undergoes polymerization with elimination of water as steam and nylon is produced in molten state. It can be then cast into sheet or fibres.
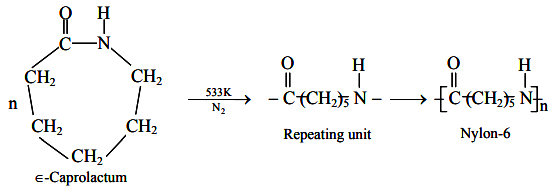
Preparation of Nylon 6 or Perlon : It can be prepared from a single monomer having a potential amino group at one end and a potential carboxyl group at the other.
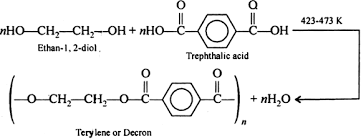
b)Polyesters
Polymers which have ester linkage are called polyesters and are prepared by condensation polymerization of diacids with diols .
Terylene or Decron is the best example of polyesters.
The fibre of terylene is highly crease resistant , durable and low moisture content. It is used for manufacture of wash and wear fabrics, type cords, seat belts.
c)Phenol – Formaldehyde Polymer
These are oldest synthetic polymer . These are obtained by condensation of phenol with formaldehyde in presence of either an acid or base as catalyst . First a linear product called novolac is obtained which is used in paints . Novolac on further heating with formaldehyde undergoes cross linking to form bakelite. Thus , bakelite is a condensation copolymer of phenol and formaldehyde. It is used in manufacture of combs , formica table tops, fountain pen barrels , electrical goods etc.
d)Melamine Formaldehyde Polymer
Melamine and formaldehyde undergo condensation copolymerization to form melamine – formaldehyde polymer also called melmac . It is widely used for making non – breakable plastic crockery , i.e., cups and plates made from melamine polymer are hardand do not break on being dropped.
Copolymerization
When two or more different monomers are allowed to polymerize together , the product formed is called a copolymer and this process is called copolymerization .
Composition of copolymers : It depends not only upon the proportion of monomers but also upon their relative reactivity. Some monomers as such do not polymerize at all but undergoes copolymerization . Example : maleic anhydride does not polymerize as such but undergoes copolymerization with styrene to form styrene – maleic anhydride copolymers.
uses of copolymers : It is used for making floor tiles , footwear components , cable insulation etc.
Rubbers
1)Natural Rubber
It is a natural polymer . It has remarkable elasticity and undergoes long range reversible extension even under relatively small applied force. Thus , it is also called elastomer. It is manufactured from latex which is colloidal solution of rubber particles in water. Latex is obtained from rubber tree.
Chemically , natural rubber is a polymer of isoprene . Since each repeating unit in polyisoprene contains a double bond , it may have either cis- or trans- orientation . Actually , natural rubber is cis-polyisoprene .
It is used in making footwear , foam mattresses, balloons , toys , rubber bands etc.
# Vulcanization
Natural rubber is soft and tacky and becomes even more so in hot weather. Its tensile strength and resistance to abrasion are low and elasticity is maintained over a low range of temperature. It is also non resistant to action of organic solvents and is also easily attacked by oxidising agents. These properties can be improved by a process called vulcanization .
Vulcanization consists of heating the raw rubber with sulphur at 373-415K . Since this process is slow additives like zinc oxide are used to accelerate the rate of vulcanization . The vulcanized rubber obtained has excellent elasticity and is resistant to action of organic solvent.
2)Synthetic Rubbers
Synthetic rubber may be defined as any vulcanisable rubber like polymer which is capable of getting stretched to twice its length . However , it returns to its original size and shape when stretching force is withdrawn . Some important synthetic rubber are as follows :
a)Neoprene
- It is a polymer of chloroprene and is also called polychloroprene.
- Chloroprene polymerises very readily (700 times faster than isoprene) . No specific catalyst is needed but poylmerisation is slow in absence of oxygen .
- It is inferior to natural rubber in some properties but superior in its stability to aerial oxidation .
- It is used in manufacture of hoses , gaskets , shoe heels , stoppers etc.
b)Buna – N
- It is obtained by copolymerization of 1,3-butadiene and acrylonitrile in presence of a peroxide catalyst.
- It is used in making oil seals , hoses and tank linings.
Molecular Mass of Polymers
Since each species has a different molecular mass and a given sample of polymer contains a number of such species , therefore the polymer as a whole has an average molecular mass . In contrast , natural polymer such as proteins, contains chains of identical lengths and have definite molecular mass.
Biodegradable Proteins
Polymers such as polysaccharides and nucleic acid which control the various life processes are called biopolymers. These can be broken down rapidly by enzyme catalysed reaction .
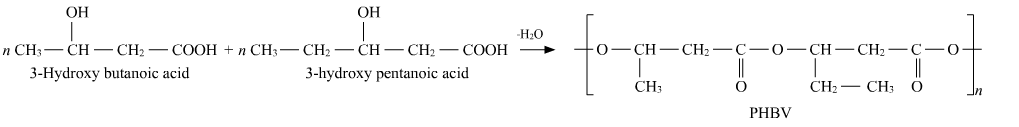
1)Poly-β-hydroxybutyrate-co-β-hydroxyvalerate ( PHBV )
It is a thermoplastic copolymer of 3-hydroxy-butanoic acid and 3-hydroxy pentanoic acid in which two monomer units are connected by ester linkage.
It is used in speciality packing and in controlling drug release.
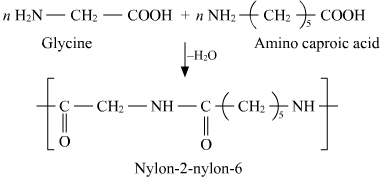
2)Nylon-2-Nylon-6
It is a copolymer of glycine and aminocaproic acid .
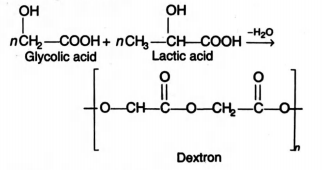
3)Dextron
It is first biodegradable polymer used as sutures, i.e., for stitching of wounds after operation . It is a copolymer of glyconic acid and lactic acid.
# Polymers Class 12
# Polymers Class 12 Notes
# Polymers Class 12 NCERT Solutions
# Polymers Class 12 Notes in Detail
Do share the post if you liked Polymers Class 12 Notes. For more updates, keep logging on BrainyLads